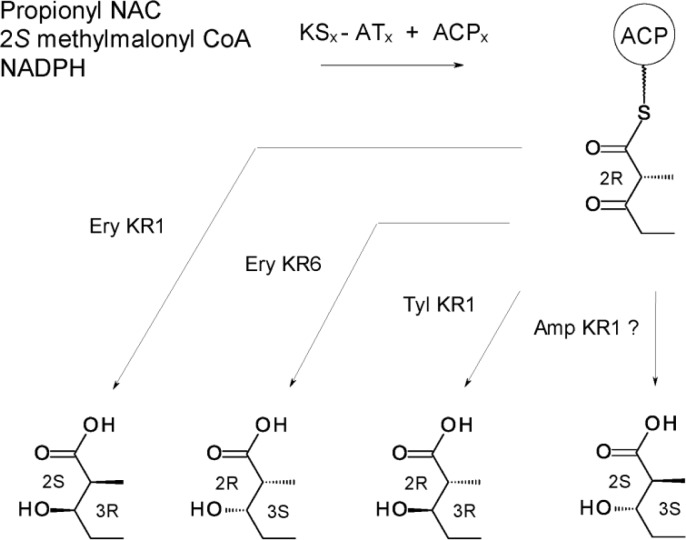
Figure 3.
In vitro synthesis of diketides. The combinations of KS-AT and ACP used were DEBS KS1-AT1 + ACP1 (epimerising), DEBS KS3-AT3 + ACP3 (epimerising), DEBS KS6-AT6 + ACP6 (non-epimerising), and PICS KS1-AT1 + PICS ACP1 (epimerising). All combinations gave a (2R)-2-methyl-3-ketoacyl-ACP initially. Enzymatic ketoreduction led to formation of a 2-methyl-3-hydroxyacyl chain with the alcohol and methyl stereochemistry characteristic of the module from which the KR was derived.