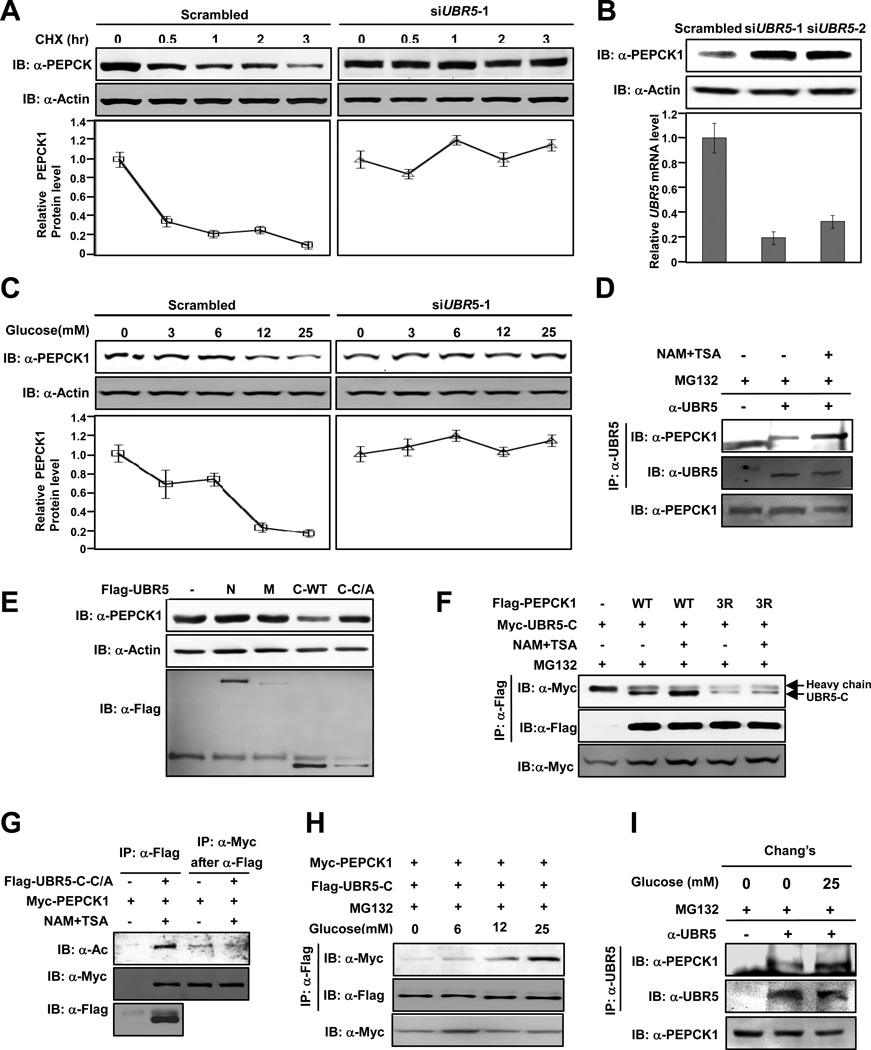
Figure 2. UBR5 is an E3 ligase of PEPCK1.
(A) UBR5 knockdown stabilizes PEPCK1. PEPCK1 levels of 293T cells with or without UBR5 knocked down were determined at different time points after CHX was added. Shown are representative western blot results of three replicates. Average quantified relative protein abundance from all three repeats is shown with SD. (B) UBR5 knockdown increases steady state PEPCK1 level. UBR5 in 293T cells was knocked down by siRNA and steady state PEPCK1 level was determined. Results of two different siRNA oligos are shown. (C) UBR5 knockdown abolishes glucose induced PEPCK destabilization. Steady state PEPCK1 levels of HEK293T cells and HEK293T cells with UBR5 knocked down were determined under different glucose concentrations. (D) Inhibition of deacetylases promotes endogenous PEPCK1-UBR5 interaction. Endogenous PEPCK1 levels co-precipitated with endogenous UBR5 under with and without MG132 treatment were detected. (E) Overexpressed UBR5-C decreases steady state PEPCK1 level. Steady state PEPCK1 levels of 293T cells were measured when UBR5 domains were overexpressed. (F) Deacetylase inhibitors affect PEPCK1-UBR5 interaction. PEPCK1-UBR5-C and PEPCK13K/R–UBR5-C interactions were determined with or without TSA and NAM treatment. (G) UBR5 binds to acetylated PEPCK1. UBR5-C-C/A and PEPCK1 were co-expressed in 293T cells. Acetylation levels of PEPCK1 associated with UBR5-C-C/A. (H) PEPCK1-UBR5 interaction is enhanced by high glucose. Myc-PEPCK1 and Flag-UBR5-C were co-expressed in 293T cells maintained at different glucose concentrations and PEPCK-UBR5-C interaction was by IP-western. (I) Glucose increase PEPCK1-UBR5 interaction. Endogenous UBR5 proteins in Chang’s cells cultured in 25mM glucose and glucose free media were precipitated and PEPCK-UBR5-C interaction was determined. See also Figure S1.