Figure 5.
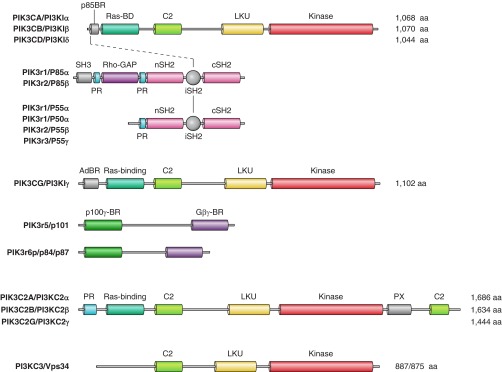
The family of PI 3-kinase enzymes. Class I PI 3-kinases phosphorylate PtdIns(4,5)P2 to PtdIns(3,4,5)P3. There are four different genes coding for the catalytic subunits of PI3Ks, called p110α, -β, -γ, and -δ. They all have a conserved COOH-terminal catalytic domain preceded by lipid kinase unique (LKU, also called “helical”), C2 and Ras binding domains (Ras-BD). These enzymes have tightly associated regulatory subunits: p110α, -β, and -δ associate with p85 and p55/50 regulatory subunits encoded by three different genes. Their association is mediated by an interaction between the very NH2-terminal p85-binding region (p85BR) of the catalytic chains with the inter-SH2 (iSH2) region of the regulatory subunits. These enzymes are also called class IA enzymes. The catalytic p110γ differs from the previous forms (hence it is called class IB) in that it associates with either of two adaptors, p101 and p84/p87. These different adaptors interact with NH2-terminal adaptor binding region (AdBR) of p110γ and lend regulation by G protein βγ subunits (p101) and perhaps by Ras (p84/p87) to the enzyme. Class II PI3Ks also come in three forms (α, β, and γ) and contain phox-homology (PX) and C2 domains placed COOH terminally to their catalytic domains. These enzymes can phosphorylate PtdIns and PtdIns4P in vitro, but their in vivo substrate preference is still debated. The enzymes most likely form PtdIns3P in the cell. The single class III PI3K and its yeast ortholog, Vps34p, phosphorylates PtdIns to PtdIns3P. These enzymes associate with a larger regulatory protein Vps15p in yeast and p150 in mammalian cells (not shown).
