Figure 9.
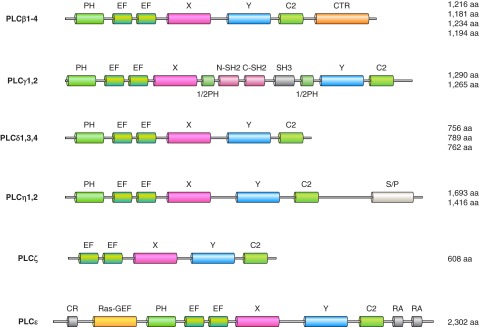
The phospholipase C family. These enzymes hydrolyze PtdIns(4,5)P2 to Ins(1,4,5)P3 and diacylglycerol, hence are called phosphoinositide-specific (PI)-PLCs. They should not be mistaken for the bacterial PLCs that are specific to PtdIns, unable to hydrolyze phosphorylated PtdIns, and hence called PI-specific PLC (unfortunately similarly abbreviated to PI-PLC). Mammalian PLCs have a core structure consisting of a PH domain, followed by EF hands, a catalytic domain formed from X and Y conserved regions and a C2 domain. PLCδ enzymes show this minimal domain organization. The only exemption is PLCζ that lacks the PH domain. This basic core is then extended in the various PLCs: the β enzymes have a characteristic COOH-terminal extension (CTR) that lends regulation by heterotrimeric G protein alpha subunits. The X and Y domains are separated with a long insert in the PLCγ enzymes consisting of two SH2 domains, an SH3 domain, and this whole insert is sandwiched in between two half PH domains. The PLCη enzymes have a long serine-proline-rich (S/P) segment in their COOH termini. The largest PLC enzyme is PLCε, which has a cysteine-reach region (CR) and a RAS-GEF domain at the NH2 terminus and two Ras association domains (RA) at the COOH terminus.
