Fig. 1.
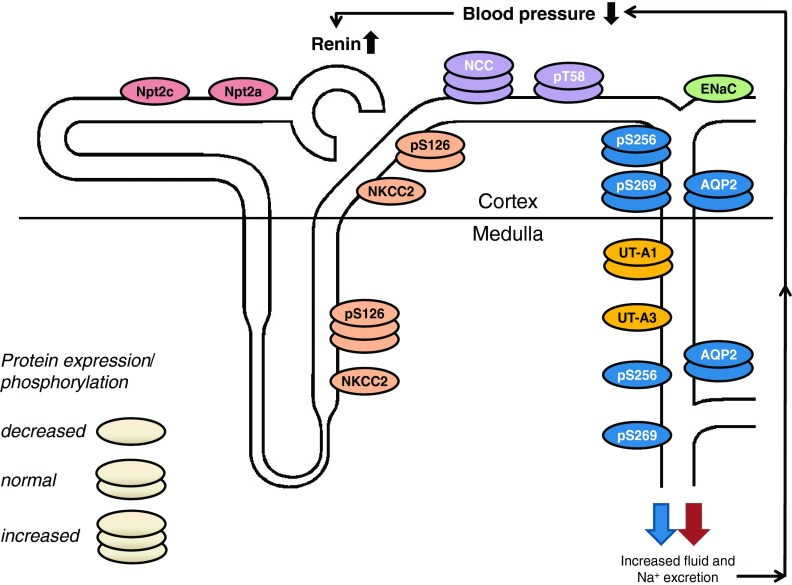
Integrated renal and blood pressure phenotype of AC6 knockout mice. AC6 may stimulate total renal Na+-K+-2Cl− cotransporter (NKCC2) protein abundance in medullary and cortical thick ascending limb (although the latter is controversial). Increased AC6-independent phosphorylation of NKCC2 at serine 126 (S126) in the medulla might help to stabilize NKCC2 activity in the absence of AC6. Upregulation of Na+-Cl− cotransporter (NCC) in AC6 knockout mice may compensate for reduced NKCC2 activity. In the aldosterone-sensitive distal nephron, the expression, but not single channel activity, of the epithelial sodium channel (ENaC) is reduced in AC6 knockout mice under basal conditions, while AVP-stimulated ENaC single-channel activity is absent. Total cortical and medullary aquaporin-2 (AQP2) were not different between genotypes; however, in the medullary portion of the collecting duct, phosphorylation of AQP2 at S256 and S269 (the latter only found in the apical plasma membrane) was severely reduced. In contrast to the medulla, cortical AQP2 phosphorylation was comparable to wild-type mice, indicating that possibly a different AC isoform regulates vasopressin-mediated water transport in the cortex. Impaired renal NaCl and fluid absorption decreases blood pressure and increases plasma renin concentration. AC6 knockout mice have comparable urinary urea excretion; however, inner medullary UT-A3 protein expression is reduced. The expression of proximal tubular Na+-phosphate transporters Npt2a and Npt2c are also severely reduced (unpublished observations), and AC6 knockout mice have phosphaturia (64). Possibly as a consequence of intact feedback mechanisms in AC6 knockout mice, renal resistance to vasopressin and parathyroid hormone causes both hormones to be significantly elevated. In summary, AC6 knockout mice have nephrogenic diabetes insipidus and a mild Bartter syndrome. See the text for a detailed description.