Fig. 1.
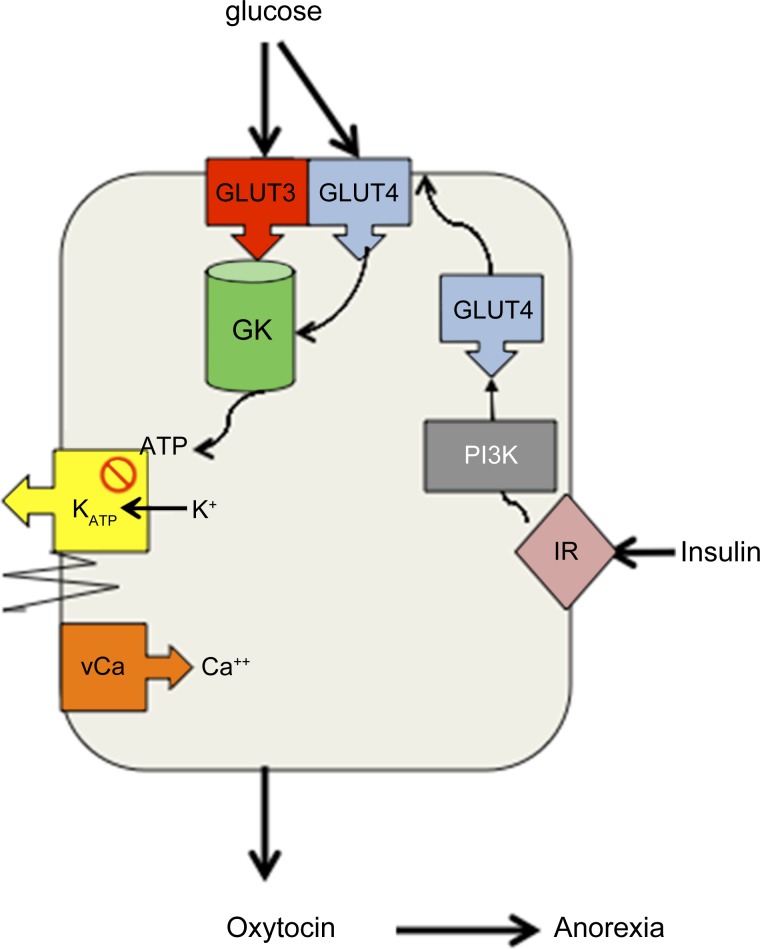
Do magnocellular neurons (MNCs) function as glucose and metabolic sensors? Expression of insulin receptors (InsR) and glucokinase (GK) by supraoptic MNCs suggests that MNCs can modulate release of the anorexigenic agent, oxytocin (OT), in response to changes in circulating insulin and the extracellular glucose concentration. We postulate that since glucose transporter 3 (GLUT3), the “brain glucose transporter,” is saturated at physiological glucose concentrations, glycolysis of glucose by GK, a hexokinase that is not inhibited by its own product (34), provides a glucose concentration-dependent source of ATP. GLUT 3 and GK are present in supraoptic nuclei (SON) (45, 75). In other glucose-sensing cells [e.g., pancreatic β-cells and ventromedial nucleus (VMN) neurons], ATP closes ATP-sensitive K+ channels (KATP) channels resulting in membrane depolarization, opening of voltage-sensitive calcium channels (vCa), and initiation of exocytosis or generation of actions potentials. Kir6.1 and 6.2 (KATP channels) are present in SON (14, 74). Thus we postulate that GK-mediated ATP production inactivates (closes) KATP channels inducing depolarization, opening of vCa, and propagation of action potentials to the nerve terminals in posterior pituitary and other central nervous system regions to initiate exocytosis of OT. We also postulate that insulin mediated membrane insertion of GLUT4, which is PI3K dependent (9, 47, 48), provides additional substrate for GK-mediated glycolysis thus further promoting KATP-mediated depolarization and OT release.