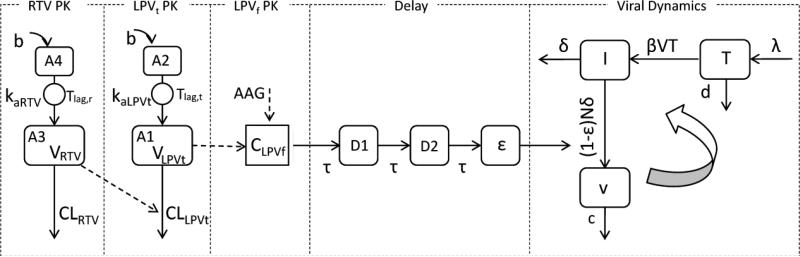
Figure 1.
Structure of the final integrated pharmacokinetic-pharmacodynamic model defined by Eqs. (1-11). (RTV: ritonavir; LPVt: total lopinavir; LPVf: free lopinavir; b: bolus input; A1: central compartment of LPVt; A2 depot compartment of LPVt; A3: central compartment of RTV; A4: depot compartment of ; CLLPVt: Clearance of LPVt with ritonavir effect; RTV; CLRTV: clearance of RTV; VRTV: volume distribution of RTV central compartment; VLPVt: volume distribution of LPVt central compartment; CLPVf: concentration of LPVf; kaLPVt: absorption rate constant of LPVt; kaRTV: absorption rate constant of RTV; Tlag,t: absorption lag time of LPVt; Tlag,r: RTV absorption lag time; AAG: α-1-acid glycoprotein; CLPVf: free lopinavir concentration; D1 and D2: delay compartments. mean transit time; T: CD4+ T-cells; I: infected CD4+ T-cells; v free virions; λ the rate of new T cells are generated in the body; d the death rate of T cells; β the infection rate; δ the death rate of infected CD4+ T-cells; c: the clearance rate of free virions; N: the number of new virions produced by each infected CD4+ T-cell during its lifetime; ε the drug inhibit efficacy.