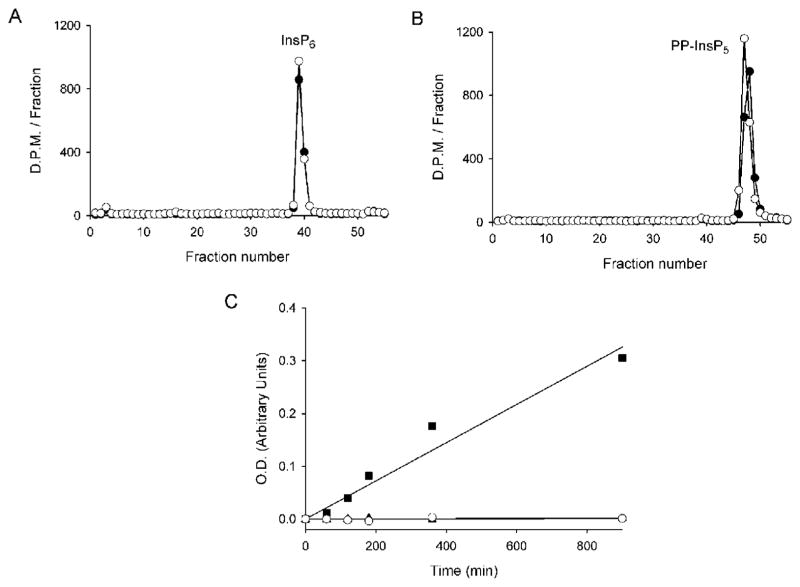
Figure 2. The phosphatase-like domains of PPIP5Ks do not have phosphatase activity.
PBD1 (6 μg) was incubated with approx. 1500–2000 d.p.m. of either [3H]InsP6 (A) or 5-PP-[3H]InsP5 (B) in 120 μl of phosphatase assay buffer for 15 h at 37 °C (closed circles). Controls (open circles) did not contain enzyme. (C) 50 mM p-nitrophenyl phosphate replaced the inositol phosphates, and the assays contained 90 μg of either PBD1 (open circles) or PBD2 (closed triangles; these symbols are largely hidden by the open circles). Reactions were quenched with 1M NaOH and the A450 indicates the accumulation of p-nitrophenol. The closed squares represents the hydrolysis of p-nitrophenylphosphate by 90 ug of hMIPP. O.D., optical density (absorbance).