Figure 5. Evidence from multiple sequence alignments that PBD1 and PBD2 are unusual hybrid PtdIns(3,4,5)P3-binding domains.
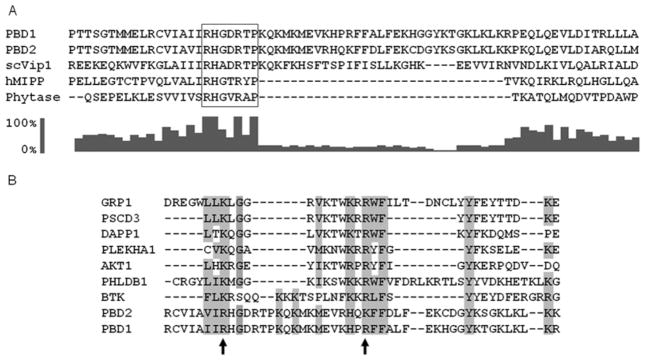
(A) Previous molecular modelling studies [10] have identified residues 530–1047 as a phosphatase-like region of Vip1 from Saccharomyces cerevisiae (scVip); the N-terminus of this domain was aligned (using ClustalX) with corresponding residues in the PBDs of PPIP5K1 (residues 382–453), PPIP5K2 (residues 371–442), hMIPP and the E. col. phytase. The RHGXRXP acid-phosphatase motif in the PBDs is boxed. The bar graph (generated with ClustalX 2.0.10) shows the conservation score (on a scale of 0 to 100 %) for each residue in the alignment. (B) A separate ClustalX sequence alignment of PBD1, PBD2 and several PtdIns(3,4,5)P3 -specific PH domains (GRP-1, PHLDB1 and PSCD3 are from mice; DAPP1, PLEKHA1, AKT1 and BTK are human sequences). The arrows mark two conserved basic residues in PBD1 (Arg399 and Arg417) that were selected for mutagenesis studies. Other conserved amino acid residues that are identical or similar in nature have also been highlighted. GenBank® accession numbers: scVip1, NP_013514; hMIPP, AAH32504.1; Phytase, 9954897; PBD2, NP_056031.2; PBD1, AAH57395.1; GRP-1, AAB60876.1; PSCD3, EDL19040.1; DAPP1, AAH12924.1; PLEKHA1, AAH01136.1; AKT1, AAL55732.1; PHLDB1, AAH69853.1; BTK, NP_000052.1.
