Figure 3.
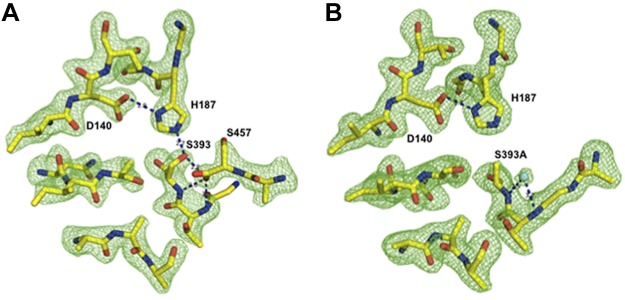
Active site comparison of WT and S393A mutant EpiP. Ball-and-stick models of the catalytic triad (D140, H187, and S393) and selected residues from the active site are presented. To indicate the high quality of the refinement and the final models, Fo-Fc omit maps (green) contoured at the 3σ level for the WT (A) and mutant (B) proteins are shown. In the active site of the WT protein, S457 of the C terminus of a neighboring WT protein molecule is bound in the active site. In the mutant protein, a water molecule resides in the same location as one of the oxygen atoms of the C-terminal carboxyl group of S457. Hydrogen bonds are shown by black dashes, nitrogen atoms are shown in blue, oxygen atoms in red, carbon atoms in yellow, and water in cyan.
