Figure 4.
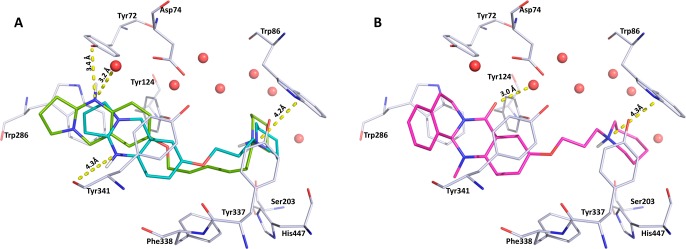
(A) Docking pose showing the inverted binding mode of inhibitor 41 (cyan) with a 4.2 Å cation−π interaction to Trp86 and potential hydrogen bonds to a water molecule (3.2 Å) and Tyr72 (3.4 Å). Inhibitor 42 (green) shows the same placement of the piperidine ring near the CAS (4.2 Å to Trp86) and a distance of 4.3 Å to the Tyr341 backbone carbonyl. (B) Selected docking pose of the tetracyclic inhibitor 21 (pink, R-enantiomeric form) showing the inverted binding mode. The inhibitor is stabilized by a 3.0 Å interaction of the scaffold carbonyl oxygen with a water molecule and a 4.3 Å cation−π interaction of the piperidine with Trp86 near the CAS. Color code: red, conserved water molecules; gray, hAChE binding site; catalytic active site, Ser203, His447 (Glu334 not shown here); peripheral anionic site (PAS), Tyr72, Trp286. Both figures were created using Pymol.35
