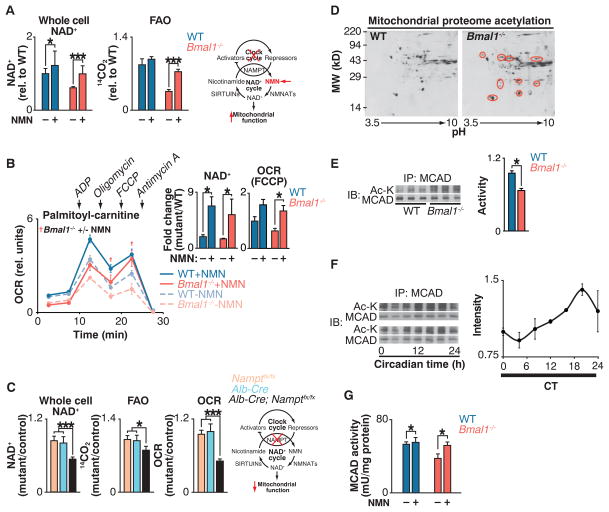
Fig. 2. Clock control of NAD+-dependent mitochondrial metabolism and protein acetylation.
(A) NAD+ and FAO in liver of WT and Bmal1−/− mice injected with either saline or NMN (250 mg/kg) 12 hours before killing at ZT0 (n = 6). Schematic shows interaction of clock with NAD+ salvage pathway, where the intermediate NMN bypasses the Nampt defect in circadian mutants. (B) OCR with palmitoyl-carnitine as substrate in mitochondria isolated from liver of fasted WT and Bmal1−/− mice that had been injected once a day for 10 days with either saline or NMN (500 mg/kg) before killing at ZT0 (n = 3). Inset: Whole-cell NAD+ after 10 days of NMN injections and quantification of uncoupled (FCCP) OCR. †P < 0.05 indicates Student’s two-tailed t test comparison between Bmal1−/− mitochondria with and without NMN treatment. (C) Relative NAD+, FAO, and palmitoyl-carnitine OCR in livers of liver-specific Nampt−/− animals (n = 3 to 6). (D) Two-dimensional gel electrophoresis of liver mitochondrial proteome from WT and Bmal1−/− mice at ZT0. Red circles represent acetylated proteins. (E) Relative MCAD acetylation and activity in WT and Bmal1−/− fasted liver mitochondrial extracts at ZT0 (n = 6). (F) Relative MCAD acetylation measured every 4 hours for 24 hours in fasted wild-type mice kept in constant darkness (n = 4). (G) Relative MCAD activity in liver of WT and Bmal1−/− mice injected with either saline or NMN (250 mg/kg) 12 hours before killing at ZT0 (n = 6). *P < 0.05, ***P < 0.001 for Student’s two-tailed t test comparing single time points between WT and mutant averages. Data are represented as average ± SEM.