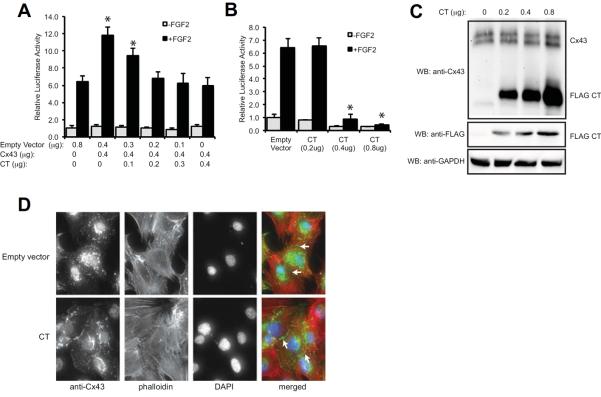
Fig. 4.
The Cx43 CT antagonizes the ability of Cx43 to stimulate Runx2 activity in MC3T3 cells. (A) Luciferase reporter assays were performed on MC3T3 cells co-transfected with a Runx2-reponsive luciferase reporter (p6xOSE2-Luc) and pSFFV-neo (empty vector) or a full-length pSFFV-Cx43 (Cx43) and increasing concentrations of pCMV3XFLAGm43CT plasmid, which encodes amino acids 236–382 of the Cx43 CT, at the indicated concentrations. Subsequently the cells were treated with or without FGF2 (10ng/ml, 4h). Data are shown as means ± s.d. *, P<0.05, relative to the corresponding FGF2-treated empty vector control. (B) Luciferase reporter assays were performed on MC3T3 cells co-transfected with a Runx2-reponsive luciferase reporter (p6xOSE2-Luc) and increasing concentrations of pCMV3XFLAGm43CT plasmid without the transfection of exogenous full-length Cx43. Subsequently the cells were treated with or without FGF2 (10ng/ml, 4h). Data are shown as means ± s.d. *, P<0.05, relative to the corresponding FGF2-treated empty vector control. (C) Western blots from transfected MC3T3 cells transfected with increasing concentrations of pCMV3XFLAGm43CT plasmid. The blots were probed with anti-Cx43, anti-FLAG and anti-GAPDH (load control) antibodies. (D) MC3T3 cells were transiently transfected with 0.8μg pCMV3XFLAGm43CT plasmid followed by immunofluorescent labeling with anti-Cx43 antibodies (green). Phalloidin (red) and DAPI (blue) staining were also performed. Arrows show labeling at cell-to-cell junctions. A representative image is shown.