Scheme 1.
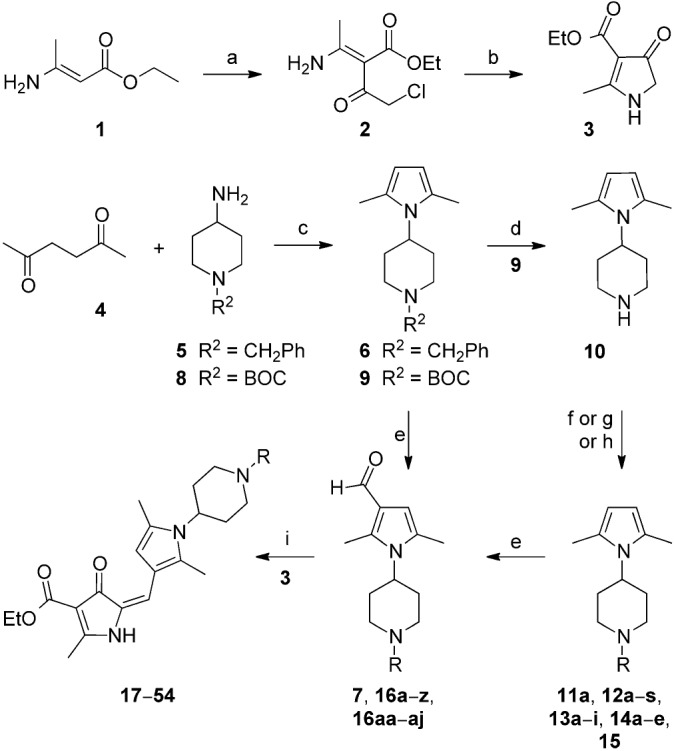
General strategy for the synthesis of piperidine derivatives. Regents and conditions: a) chloroacetyl chloride, pyridine, 0 °C, 30 min, 75 % yield; b) KOH, EtOH, 3 h, 0 °C, 95 % yield; c) p-TsOH bound on silica gel, microwave (0–400 W at 2.45 GHz), 180 °C, 15–20 min, 80–90 % yield; or p-TsOH, toluene, Dean–Stark apparatus, 90 °C, 3 h, 80–90 %; d) 4 m HCl/dioxane, 0 °C, 3 h, 66 % yield; e) POCl3, DMF, 100 °C, 3 h, 80–95 % yield; f) ROSO2CH3, Et3N, NaHCO3, CH3CN, 85 °C, 12 h, 50–70 %; g) RBr, DIPEA, DMF, 85 °C, 3 h, 40–70 % yield; h) alkyl/aryl/heterocyclic aldehyde, sodium triacetoxyborohydride, MeCN, RT, 8 h, 60–80 % yield; i) KHSO4, EtOH, 3 h, reflux, 80–95 % yield. R groups are given in Table 1.
