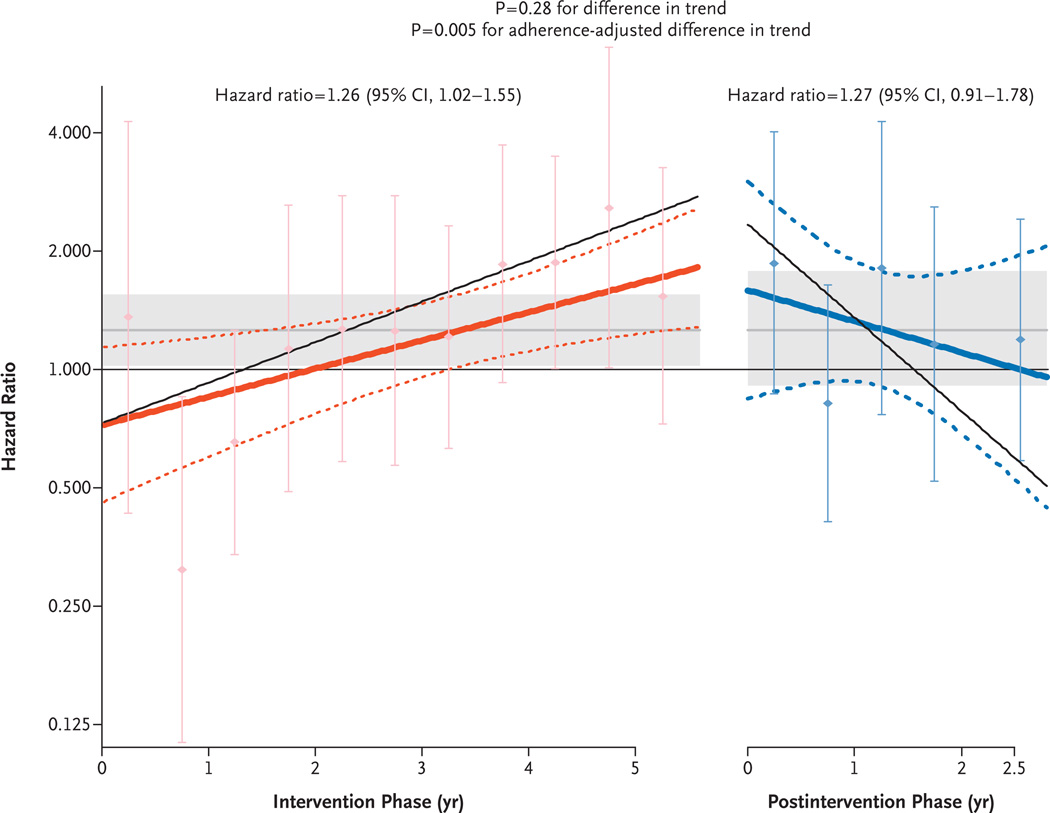
Figure 1. Effects over Time of Estrogen plus Progestin on the Incidence of Breast Cancer in the WHI Clinical Trial.
Time-varying linear hazard ratios and 95% confidence intervals (solid and dashed lines, respectively) are shown for the effect of conjugated equine estrogens plus medroxyprogesterone acetate on the risk of breast cancer as compared with placebo during the intervention and postintervention phases of the study. The shaded areas indicate the overall mean and 95% confidence intervals for the hazard ratios in the intervention and postintervention phases. The I bars show hazard ratios and 95% confidence intervals according to an analysis based on events accumulated at 6-month intervals. The P value of 0.28 for a difference in trend is for the comparison of the hazard-ratio slopes in the two study phases in the primary, unadjusted analysis, and the P value of 0.005 is for a difference in trend from an analysis adjusted for adherence status, with censoring of events that occurred 6 months after a woman became nonadherent (defined as consuming <80% of study pills or starting hormone therapy). The thin solid lines show the adherence-adjusted, time-varying linear hazard ratios.