Abstract
Background
High-resolution manometry and esophageal pressure topography have ienhanced our ability to analyze esophageal motor disturbances by improving the detail and accuracy of measurements of peristaltic activity. This has been extremely helpful in the evaluation of disorders of rapid propagation as the technique is able to define important time points and physiologic landmarks that are crucial in defining peristaltic velocity and latency intervals.
Purpose
The goal of the current review will be to assess how esophageal pressure topography has impacted our ability to define important phenotypes of rapid propagation. Additionally, this review will also be utilized to complement the description of the Chicago Classification of Esophageal Motor Disorders, which is presented in this supplement issue.
Introduction
High resolution manometry (HRM), in and of itself, is an adaptation of conventional manometric hardware that basically incorporates an increased number of pressure sensors spaced closely together. The data generated by HRM would therefore be displayed as a tracing format similar to what would be utilized for conventional manometric interpretation. The real advance in terms of manometry is primarily focused on the analysis techniques that were derived to optimize the information from high-resolution manometry. In order to better visualize the data, Clouse and Staiano incorporated a process of interpolation or averaging between sensors to display the information in the form of seamless isobaric color regions on esophageal pressure topography plots (EPT) (1) (Figure 1). The EPT or “Clouse Plots” have the capacity to convert manometric information into distinct patterns that illustrate the physiology of contractile coordination and the mechanics associated with bolus transit evidenced on combined studies with fluoroscopy and impedance
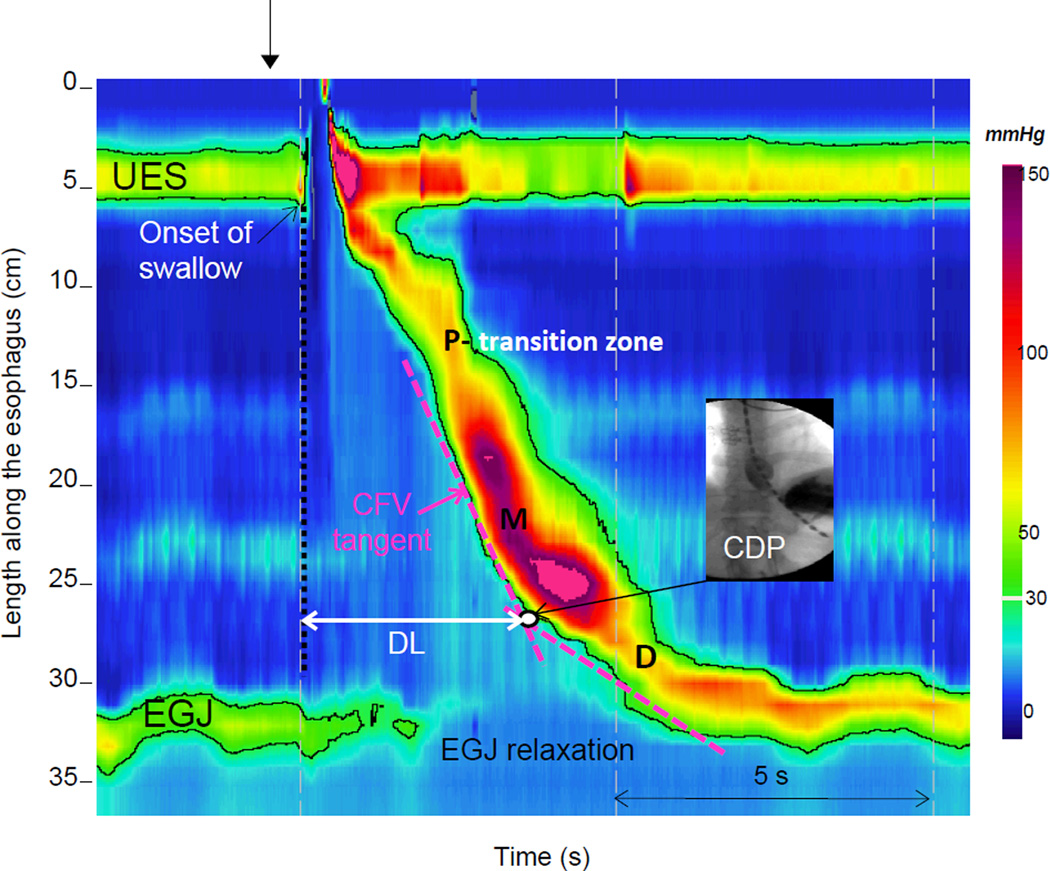
Figure 1.
A normal swallow in a Clouse plot. Two high pressure zones are visualized: the upper esophageal sphincter (UES) and the esophago-gastric junction (EGJ). The highlighted black line is the 30-mmHg isobaric contour circumscribing areas with pressure greater than 30 mmHg. The peristaltic esophageal contraction is characterized by 3 troughs, one proximal (P), one middle (M) and one distal (D). Defects in the proximal trough are labeled as transition zone defects while defects in the middle or distal trough can be labeled as being associated with ineffective esophageal motility due to the proximity of the troughs to the 3 and 8cm locations above the EGJ. The Contractile Deceleration Point (CDP) represents the inflexion point in the contractile front propagation. It is localized on Clouse plots by fitting 2 tangential lines to the initial and terminal portions of the 30-mmHg isobaric contours and noting intersection of the lines (white dot). On fluoroscopic image it corresponds to the transition to ampullary conformation and slowing of the luminal closure front. The Contractile Front Velocity (CFV) corresponds to the slope of the tangent line to the initial portion of the contraction (between P and the Contractile Deceleration Point). The distal latency (DL) is measured from the onset of swallow (dashed vertical line) to the Contractile Deceleration Point. Modified from the HRM Working Group.
This new technique has benefitted our evaluation of esophageal body peristaltic function by improving both the detail and accuracy of measurements of peristaltic function. First, important landmarks can be described that impact measurements of integrity of the wavefront and the pattern of propagation. These landmarks; coupled with the ability to define a space-time domain, greatly improves our ability to define the timing of contractile events and bolus transit through the esophagus and into the stomach. A disruption in the sequencing and order of contraction, as it relates to the timing of EGJ opening, will impair bolus transit and potentially lead to complications, such as discomfort and regurgitation/aspiration. The goal of this manuscript will be to detail how EPT has impacted our ability to measure important components of peristaltic function with a focus on contractile propagation and spastic disorders of the esophagus. Although we will discuss both the physiology and quantitative metrics utilized for assessing contractile vigor, the assessment of hypercontractility and weak peristalsis will be covered in greater detail in other sections of this supplement.
Esophageal Body Motility
Swallowing not only induces a contraction wave that progresses down the esophageal body but also triggers a wave of inhibition of the esophageal smooth muscle that precedes the arrival of the peristaltic contraction (deglutitive inhibition), resulting in relaxation of the lower esophageal sphincter and in preparation of the esophageal body to receive the oncoming bolus with minimal distal resistance(2, 3). Experiments in vitro and in vivo have shown that a wave of muscle hyperpolarization spreads down the esophageal body preceding the occurrence of peristaltic contractions(4–7). The initial hyperpolarization of the muscle lasts progressively longer in progressively more distal segments; hence it may play an important role in the normal propagation of primary peristalsis(4).
The pattern of activation of the inhibitory and excitatory vagal pathways, the regional gradients of inhibitory and excitatory myenteric nerves, and the intrinsic properties of the smooth muscle all determine the latency between swallow and contractions and the velocity of peristalsis. The esophageal peristaltic contractions themselves are a blend of noncholinergic and cholinergic components. As a consequence, cholinergic antagonists, such as atropine, increase the latency and decrease the amplitude of contraction in the proximal but not the distal parts of the esophagus. In contrast, antagonists of nitric oxide synthase reduce the latency mainly in the distal segments and lead to simultaneous contractions.
Any condition in the gastrointestinal tract which impairs neural inhibitory activity will probably result in a discoordinated motor behavior. In the esophagus, the classical example of degenerative loss of neurons and impairment of inhibitory activity is achalasia of the LES. In 1970 Christensen suggested that a system of local inhibitory innervation was crucial to the understanding of the pathogenesis of diffuse esophageal spasm and achalasia(8). Evidence of inhibitory dysfunction associated with abnormal peristalsis and/or incomplete LES relaxation has been found both in animal experiments(9, 10) and in patients with achalasia(11–14). Since then many investigators proposed that the spectrum of primary esophageal motility disorders may be due to different degrees of inhibitory dysfunction(8, 15–18).
Given the fact that deglutitive inhibition and latency intervals are crucial in determining propagation of contractile activity in the esophagus, new metrics focused on defining latency and contractile velocity have been devised using EPT. The clear description of the timing of important events with EPT, such as the onset of swallowing and the contractile deceleration point, have allowed a more uniform description of latency and velocity. These new measurements have been integrated with other measurements focused on the integrity and vigor of contraction to determine phenotypes of rapid propagation that may be both clinically relevant and physiologically distinct.
Esophageal Pressure Topography
Anatomy and Landmarks
Figure 1 depicts the typical pressure topography of both sphincters and the intervening esophagus during a normal swallow. Clouse originally described three types of pressure troughs in the topography of peristalsis, labeled P (proximal), M (middle), and D (distal)(19). Although the clinical relevance and neuromuscular control of these segments is unclear, swallows may also exhibit exaggerated troughs with defects in the 20 mmHg isobaric contour. Defects within the proximal trough had previously been labeled a transition zone defect based on the hypothesis that this area represented a transition in the neuromuscular control of the esophagus from extrinsically controlled striated muscle to intrinsically dominated smooth muscle. Defects in this particular location which are greater than 2cm using a 20 mmHg isobaric contour can be associated with impaired bolus transit and proximal stasis (20–22). In contrast, defects associated with the middle and distal pressure troughs are more in line with what would be defined as ineffective esophageal motility given the proximity of the middle and distal trough with the 3 to 8 cm domain above the LES utilized in defining this entity.
In addition to the segmental architecture displayed by the Clouse Plots, another important landmark becomes apparent that has important implications in the context of assessing propagation. The contractile deceleration point (CDP) is defined as the point where the most abrupt deceleration in velocity occurs within the distal esophagus (23). This landmark defines a transition from esophageal peristaltic clearance to emptying of the phrenic ampulla on fluoroscopy where the mechanism of emptying is dominated by compartmentalized pressurization, re-elongation and recoil of the phrenoesophageal ligament rather than peristaltic propagation (23). The Contractile Deceleration Point location is determined by defining the intersection point between two tangent lines of the 30 mmHg isobaric contour: one extending distally from the transition zone and the other extending proximal from the EGJ when it reestablishes its normal post-deglutitive position (Figure 1). The distance of the Contractile Deceleration Point above the proximal margin of the EGJ typically ranges from 1.5 to 2cm and thus, the previous landmarks to assess peristalsis using 3 and 8cm above the LES, were in fact a reasonable estimate of peristaltic function.
Assessing Propagation using Esophageal Pressure Topography Metrics
The assessment of peristaltic integrity should always be the first step in the evaluation of peristaltic function as the integrity of the contraction will determine whether other measurements are feasible or worthwhile (Table 1). The integrity of the contraction using a 20 mmHg isobaric contour associated with each swallow describes how completely that contraction spans from the upper sphincter to the EGJ, irrespective of the vigor or velocity of the contraction. Qualifying measures of Distal Latency and Contractile Front Velocity can only be measured accurately when there is sufficient contractile integrity to allow proper landmark identification and measurement.
Table 1.
Esophageal pressure topography metrics utilized in assessing propagation.
| Pressure Topography Metrics | |
|---|---|
|
Measures focused on Anatomy and Landmarks Must be defined before qualifiers can be measured | |
Peristaltic Integrity
|
Peristaltic breaks: (cm) Gaps in the 20 mmHg isobaric contour of the peristaltic contraction between the UES and EGJ, measured in axial length.
Failed swallow: integrity of the 20 mmHg isobaric contour distal to the proximal pressure trough (P) measures less than 3 cm in length |
Contractile Deceleration Point
|
The inflection point along the 30 mmHg isobaric contour where propagation velocity slows demarcating the tubular esophagus from the phrenic ampulla.
|
|
Qualifiers of Contractile Vigor and Propagation Should not be measured in failed or swallows with > 5 cm breaks in the distal pressure trough | |
Contractile Front Velocity
|
Slope of the tangent approximating the 30 mmHg isobaric contour between transition zone (P) and the CDP
|
Distal Latency
|
Interval between UES relaxation and the CDP
|
Distal Contractile Integral
|
Uses a space time box to measure amplitude x duration x length (mmHg-s-cm) of the distal esophageal contraction greater than 20 mmHg from proximal (P) to distal (D) pressure troughs.
|
Defects or breaks in peristaltic activity can be severe to the point where no propagating contractile activity is present or only a short segment of contraction defined by a 20 mmHg isobaric contour less than 3 cm is noted(24, 25). These swallows are defined as failed and therefore, Distal Latency or Contractile Front Velocity should not be measured. Defects are further defined based on size of the breaks [large >5 cm but not failed, small 2–5 cm](25). Large defects noted in the distal esophagus will potentially conceal the Contractile Deceleration Point and thus, Distal Latency and Contractile Front Velocity may be difficult to interpret. These swallows are more akin to swallows labeled as ineffective esophageal motility (IEM) as they will have peristaltic amplitudes below 30 mmHg in a pressure sensor placed 3–8 cm above the EGJ. Therefore, one would define these swallows based on the defect in integrity as a weak swallow and a determination of abnormal propagation will not be possible.
Small breaks in the peristaltic wavefront may not obscure measurement of the the Contractile Deceleration Point, and thus Distal Latency and Contractile Front Velocity can be measured when these defects are not localized at the middle or distal trough. Defects occurring at the proximal trough or transition zone should not complicate the determination of the CDP and will not alter the accuracy of measuring Distal Latency and Contractile Front Velocity(26).
Velocity
The measurement of peristaltic velocity is the primary critieria for diagnosing spasm using conventional manometry based on the concept that a velocity greater than 7.5 to 8 cm per second is synonymous with a simultaneous contraction(27). However, esophageal pressure topography has revealed that velocity along the contractile wavefront is dependent on contractile morphology and regional changes in distal propagation. This is most notable in the description of the Contractile Deceleration Point which describes a clear demarcation of two distinct areas of functional activity focused on changes in velocity along the propagating wavefront. Propagation of the contractile wavefront beyond the Contractile Deceleration Point is not peristaltic as it represents emptying of the phrenic ampulla. Therefore, a more accurate assessment of peristaltic velocity is made by taking the best-fit tangent between the transition zone and Contractile Deceleration Point. This is in contrast to the arbitrary utilization of 3 and 8cm above the EGJ where the assessment of peristaltic velocity may extend into the phrenic ampulla.
The Contractile Front Velocity is calculated by taking the best-fit tangent between the transition zone and Contractile Deceleration Point (Figure 1). In instances of increased intrabolus pressure, trapped between the distal esophageal contraction and the EGJ, the Contractile Front Velocity should be determined at an isobaric contour pressure exceeding EGJ pressure so as not to mistake esophageal pressurization for a simultaneous contraction (28). The upper limit of normal for the Contractile Front Velocity is > 9 cm/s based on 95th percentile values of data in 75 normal subjects (23).
Latency
The Distal Latency is a measure that is reflective of peristaltic timing and the period of deglutitive inhibition rather than the late phase of esophageal emptying. The measurement is made by taking the time from the start of upper esophageal sphincter (UES) relaxation to the Contractile Deceleration Point and represents a means for quantifying the latency of the distal contraction as a surrogate for inhibitory ganglionic integrity (29) (Figure 1). A computer simulation using normalized measurements of esophageal length determined that the median Distal Latency was 6.0 s (95% CI 4.8–7.6 s) (30) A manual analysis of the same data, found that the median time for propagation from the start of UES relaxation to the Contractile Deceleration Point was 6.2 seconds with a minimal value observed of 4.6 seconds in the control group. Given these findings, a cut-off of 4.5 s was established as the lower limit of normal for the Distal Latency. This threshold is also similar to the values previously described by Behar and Biancani with a small shift to account for the difference in landmarks for the beginning of the swallow (29). Although Distal Latency can be measured with conventional manometry, it is difficult to standardize because of gaps in pressure sensor spacing and poor identification of the segmental boundaries of the contraction.
Contractile Vigor
Although contractile vigor is an important measure of overall peristaltic function, it does not have a significant impact on defining the timing and coordination of the contractile wavefront. That being said, abnormalities of contractile vigor may represent an imbalance in the neurologic input of the smooth muscle esophagus and thus, could potentially define relevant pathology. The EPT metric devised to characterize the vigor of the distal esophageal contraction is the Distal Contractile Integral (DCI), measured for the segment spanning from the proximal to distal pressure troughs (31). To exclude the effects of intrabolus pressure in the DCI computation, the first 20 mmHg is ignored (31). The upper limit of normal defined by the 95th percentile in a normal population is 5,000 mmHg-s-cm (31).
The conventional criteria of distal esophageal spasm utilized a cut-off threshold value of 30 mmHg to distinguish contractile activity from non-contractile pressure events in the esophageal body(27). Similarly, EPT does require a minimum contractile activity that is defined by assessing the peristaltic integrity using the 20 mmHg isobaric contour. The DCI was devised primarily to identify swallows of excessive contractile vigor and thus, there is no lower limit to use as a criterion for spasm and the fall back has been to utilize the peristaltic integrity as an inclusion criteria. Failed swallows, swallows with large breaks in the distal trough or the appearance of a pan-esophageal pressurization preclude measuring Distal Latency and Contractile Front Velocity as the accuracy and relevance of these measures is unclear in these swallow types.
In contrast to defining threshold contractile levels to define inclusion criteria for disorders of rapid propagation, DCI measurements above normal values may have clinical relevance. This is most apparent in the instances where the DCI value is greater than 8000 mmHg-s-cm where contractions are typically associated with repetitive contractions that may extend beyond the timing of the normal deglutitive swallow window(28). These swallows may represent a disorder of altered neurogenic input as the termination of the contraction is abnormal. These patterns of hypercontractility can be seen in patients with and without abnormal propagation of the contractile wavefront and the clinical relevance of these contractile patterns will be covered in another review in this supplement.
Phenotypes of Rapid Propagation
The classification of esophageal motor disorders associated with rapid peristaltic propagation has been refined to include a measure focused on deglutitive inhibition in addition to the standard definition focused on peristaltic velocity. The diagnosis of distal esophageal spasm using conventional criteria utilized a peristaltic velocity threshold of greater than 7.5–8.0 cm/sec as criteria for spasm with the caveat that the contractile activity was greater than 30 mmHg at the level of 3 and 8cm above the EGJ(27). However, this definition of DES identifies a very heterogeneous population as evidenced on an assessment of bolus transit in 71 such patients with combined manometry and impedance (32). Thus, new criteria utilizing EPT metrics focused on Contractile Front Velocity and Distal Latency have been proposed to improve the categorization of disorders of rapid propagation.
Although quantifying Distal Latency is not unique to EPT(33),(34), the technique simplifies and potentially standardizes this measurement. EPT provides the detail required to define the segmental architecture of peristalsis necessary to differentiate regional variations in contraction velocity. Additionally, it allows one to define the onset of the swallow and the point where esophageal contraction shifts from peristalsis to ampullary emptying. These landmarks are crucial for accurate measurement of the Contractile Front Velocity and Distal Latency.
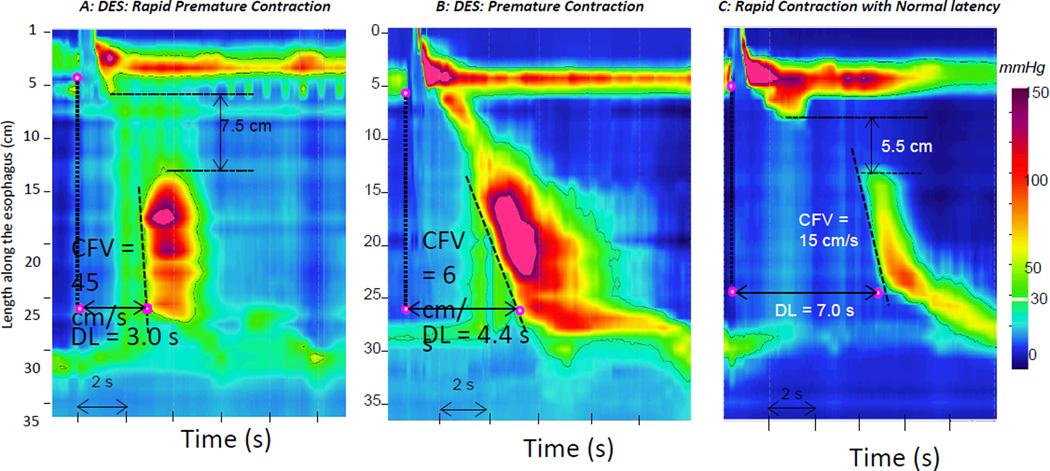
A recent study applied carefully standardized metrics for Contractile Front Velocity and Distal Latency to a large series (1070 consecutive studies) of clinical high resolution EPT studies in an attempt to refine the diagnosis of DES(26). The results defined 3 distinct subtypes of rapid propagation: 1) rapid premature contraction, 2) premature contraction and 3) rapid contraction with normal latency (Figure 2). Only 2% subjects (n=24) in that series were defined as having premature contractions [reduced Distal Latency]. This categorization is important because it is never encountered in asymptomatic control populations and these swallows will also have impaired deglutitive inhibition during multiple rapid swallows. These patients are classified as having Distal Esophageal Spasm in the Chicago Classification if there is evidence of normal EGJ relaxation (Mean Integrated Relaxation Pressure < 15 mmHg). Only 6 patients (0.5%) in this series were classified as true DES based on the criteria of reduced Distal Latency and a rapid Contractile Front Velocity in 20% or more swallows highlighting the fact that this disorder is extremely rare. Patients with reduced Distal Latency and rapid Contractile Front Velocity in the context of abnormal EGJ relaxation (Mean Integrated Relaxation Pressure < 15 mmHg) are classified as having Type III achalasia.
Figure 2.
Phenotypes of rapid propagation. Disorders of propagation are defined by two criteria: 1) Premature contraction characterized by a reduced Distal Latency (DL, measured from onset of swallow to the Contractile Deceleration Point < 4.5 s) and 2) Rapid contraction defined by a rapid Contractile Front Velocity (CFV, measured from the proximal trough to the Contractile Deceleration Point to be > 9 cm/s). Classification of Distal Esophageal Spasm (DES) requires that the contraction be premature with (Panel A) or without (Panel B) rapid contraction. Swallows associated with premature contraction will have impaired bolus transit secondary to entrapment of the bolus within the esophageal body and thus, represents a manometric correlate of the “corkscrew” or “rosary bead” esophagus. Swallows which meet criteria for Rapid Contraction with Normal Latency (Panel C) are differentiated from Distal Esophageal Spasm to highlight the overlap with normal and weak peristalsis. This swallow is associated with a large break in the proximal pressure trough which may obscure the measurement by shifting the starting point for the Contractile Front Velocity determination to the right. The bolus transit abnormality in this example is a manifestation of proximal escape through the wavefront and not entrapment which is seen with Distal Esophageal Spasm
Rapid contractions with normal latency is a much more common entity compared to premature contractions(26). In the same series of 1070 patients, 67 patients (6%) fulfilled criteria for rapid contraction with normal latency in 20% or more swallows using the Chicago Classification definition. The majority of these patients were ultimately classified as having either weak peristalsis (61%) with a proximal defect or otherwise normal/borderline normal peristalsis with normal transit mechanics. The clinical implication of rapid contraction with normal latency is unclear and probably rests within the confines of a normal variant or weak peristalsis. Interesting physiologic studies using low doses of atropine reveal that rapid contractions can be shifted to the right suggesting a potential neural cholinergic issue at the transition zone and future studies should focus on assessing the effects of atropine and nitric oxide inhibitors on these subtypes of rapid propagation.
Conclusion
Incorporating the measurement of Distal Latency into the diagnostic algorithm for assessing esophageal motor disorders has helped define distinct pathophysiologic phenotypes of rapid propagation(26). Disorders of rapid propagation are now defined into categories based on this measurement using the Chicago Classification system. Distal Esophageal Spasm and Type III achalasia are associated with premature contractions characterized by functionally impaired inhibition in the distal esophagus [reduced Distal latency]. In contrast, Rapid contraction with normal latency is a separate diagnosis highlighting preserved deglutitive inhibition and the overlap with normal and weak peristalsis. Although this classification is based on definitions extrapolated from prior physiologic investigations and the assessment of simulations in normal asymptomatic controls, the clinical relevance of this classification scheme needs to be tested in long-term natural history studies and outcome trials. We theorize that this distinction may have clinical relevance in directing therapy in that patients with reduced latency should respond better to agents that target smooth muscle relaxation (calcium channel blockers, nitrates, phosphodiesterase type 5 inhibitors). On the contrary, patients with rapid contraction and normal latency would probably do poorly with treatment focused on inducing smooth muscle relaxation and would be more suitable with a management approach similar to weak peristalsis or functional dysphagia. Further work will also require a focus on defining physiologic responses to various pharmacologic interventions and provocative swallows to determine the appropriate threshold levels and targets for treatment.
Acknowledgments
This work was supported by R01 DK079902 (JEP) from the Public Health Service and [Daniel- if there is anything you have to list]
Daniel Sifrim research work is supported by: MRC (UK) (89495); FWO (Belgium) G.0682.08 and Sandhill Scientific, USA
Footnotes
Conflict of Interest:
John Pandolfino [Given Imaging (Consulting, Educational), Sandhill (Consulting)] Daniel Sifrim [Sandhill (Research Grant)]
References
- 1.Clouse RE, Staiano A. Topography of the esophageal peristaltic pressure wave. Am J Physiol. 1991;261(4 Pt 1):G677–G684. doi: 10.1152/ajpgi.1991.261.4.G677. [DOI] [PubMed] [Google Scholar]
- 2.Vantrappen G, Hellemans J. Studies on the normal deglutition complex. Am J Dig Dis. 1967;12(3):255–266. doi: 10.1007/BF02233643. [DOI] [PubMed] [Google Scholar]
- 3.Mittal RK, Ren J, McCallum RW, Shaffer HA, Jr, Sluss J. Modulation of feline esophageal contractions by bolus volume and outflow obstruction. Am J Physiol. 1990;258(2 Pt 1):G208–G215. doi: 10.1152/ajpgi.1990.258.2.G208. [DOI] [PubMed] [Google Scholar]
- 4.Goyal RK, Gidda JS. Relation between electrical and mechanical activity in esophageal smooth muscle. Am J Physiol. 1981;240(4):G305–G311. doi: 10.1152/ajpgi.1981.240.4.G305. [DOI] [PubMed] [Google Scholar]
- 5.Rattan S, Gidda JS, Goyal RK. Membrane potential and mechanical responses of the opossum esophagus to vagal stimulation and swallowing. Gastroenterology. 1983;85(4):922–928. [PubMed] [Google Scholar]
- 6.Sugarbaker DJ, Rattan S, Goyal RK. Mechanical and electrical activity of esophageal smooth muscle during peristalsis. Am J Physiol. 1984;246(2 Pt 1):G145–G150. doi: 10.1152/ajpgi.1984.246.2.G145. [DOI] [PubMed] [Google Scholar]
- 7.Paterson WG. Electrical correlates of peristaltic and nonperistaltic contractions in the opossum smooth muscle esophagus. Gastroenterology. 1989;97(3):665–675. doi: 10.1016/0016-5085(89)90638-0. [DOI] [PubMed] [Google Scholar]
- 8.Christensen J. Pharmacologic identification of the lower esophageal sphincter. J Clin Invest. 1970;49(4):681–691. doi: 10.1172/JCI106280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gidda JS, Buyniski JP. Swallow-evoked peristalsis in opossum esophagus: role of cholinergic mechanisms. Am J Physiol. 1986;251(6 Pt 1):G779–G785. doi: 10.1152/ajpgi.1986.251.6.G779. [DOI] [PubMed] [Google Scholar]
- 10.Yamato S, Spechler SJ, Goyal RK. Role of nitric oxide in esophageal peristalsis in the opossum. Gastroenterology. 1992;103(1):197–204. doi: 10.1016/0016-5085(92)91113-i. [DOI] [PubMed] [Google Scholar]
- 11.Dent J, Dodds WJ, Hogan WJ, Arndorfer RC, Teeter BC. Effect of cholecystokinin-octapeptide on opossum lower esophageal sphincter. Am J Physiol. 1980;239(3):G230–G235. doi: 10.1152/ajpgi.1980.239.3.G230. [DOI] [PubMed] [Google Scholar]
- 12.Aggestrup S, Uddman R, Sundler F, Fahrenkrug J, Hakanson R, Sorensen HR, et al. Lack of vasoactive intestinal polypeptide nerves in esophageal achalasia. Gastroenterology. 1983;84(5 Pt 1):924–927. [PubMed] [Google Scholar]
- 13.Holloway RH, Wyman JB, Dent J. Failure of transient lower oesophageal sphincter relaxation in response to gastric distension in patients with achalasia: evidence for neural mediation of transient lower oesophageal sphincter relaxations. Gut. 1989;30(6):762–767. doi: 10.1136/gut.30.6.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holloway RH, Dodds WJ, Helm JF, Hogan WJ, Dent J, Arndorfer RC. Integrity of cholinergic innervation to the lower esophageal sphincter in achalasia. Gastroenterology. 1986;90(4):924–929. doi: 10.1016/0016-5085(86)90869-3. [DOI] [PubMed] [Google Scholar]
- 15.DiMarino AJ., Jr Characteristics of lower esophageal sphincter function in symptomatic diffuse esophageal spasm. Gastroenterology. 1974;66(1):1–6. [PubMed] [Google Scholar]
- 16.Gidda JS, Goyal RK. Regional gradient of initial inhibition and refractoriness in esophageal smooth muscle. Gastroenterology. 1985;89(4):843–851. doi: 10.1016/0016-5085(85)90582-7. [DOI] [PubMed] [Google Scholar]
- 17.Ouyang A, Reynolds JC, Cohen S. Spike-associated and spike-independent esophageal contractions in patients with symptomatic diffuse esophageal spasm. Gastroenterology. 1983;84(5 Pt 1):907–913. [PubMed] [Google Scholar]
- 18.Sifrim D, Janssens J, Vantrappen G. Failing deglutitive inhibition in primary esophageal motility disorders. Gastroenterology. 1994;106(4):875–882. doi: 10.1016/0016-5085(94)90745-5. [DOI] [PubMed] [Google Scholar]
- 19.Clouse RE, Staiano A. Topography of normal and high-amplitude esophageal peristalsis. Am J Physiol. 1993;265(6 Pt 1):G1098–G1107. doi: 10.1152/ajpgi.1993.265.6.G1098. [DOI] [PubMed] [Google Scholar]
- 20.Fox M, Hebbard G, Janiak P, Brasseur JG, Ghosh S, Thumshirn M, et al. High-resolution manometry predicts the success of oesophageal bolus transport and identifies clinically important abnormalities not detected by conventional manometry. Neurogastroenterol Motil. 2004;16(5):533–542. doi: 10.1111/j.1365-2982.2004.00539.x. [DOI] [PubMed] [Google Scholar]
- 21.Ghosh SK, Pandolfino JE, Kwiatek MA, Kahrilas PJ. Oesophageal peristaltic transition zone defects: real but few and far between. Neurogastroenterol Motil. 2008;20(12):1283–1290. doi: 10.1111/j.1365-2982.2008.01169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pohl D, Ribolsi M, Savarino E, Fruhauf H, Fried M, Castell DO, et al. Characteristics of the esophageal low-pressure zone in healthy volunteers and patients with esophageal symptoms: assessment by high-resolution manometry. Am J Gastroenterol. 2008;103(10):2544–2549. doi: 10.1111/j.1572-0241.2008.02062.x. [DOI] [PubMed] [Google Scholar]
- 23.Pandolfino JE, Leslie E, Luger D, Mitchell B, Kwiatek MA, Kahrilas PJ. The contractile deceleration point: an important physiologic landmark on oesophageal pressure topography. Neurogastroenterol Motil. 2010;22(4):395–400. e90. doi: 10.1111/j.1365-2982.2009.01443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bulsiewicz WJ, Kahrilas PJ, Kwiatek MA, Ghosh SK, Meek A, Pandolfino JE. Esophageal pressure topography criteria indicative of incomplete bolus clearance: a study using high-resolution impedance manometry. Am J Gastroenterol. 2009;104(11):2721–2728. doi: 10.1038/ajg.2009.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roman S, Lin Z, Kwiatek MA, Pandolfino JE, Kahrilas PJ. Weak peristalsis in esophageal pressure topography: classification and association with Dysphagia. Am J Gastroenterol. 2011;106(2):349–356. doi: 10.1038/ajg.2010.384. PMCID: 3035759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pandolfino JE, Roman S, Carlson D, Luger D, Bidari K, Boris L, et al. Distal esophageal spasm in high-resolution esophageal pressure topography: defining clinical phenotypes. Gastroenterology. 2011;141(2):469–475. doi: 10.1053/j.gastro.2011.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spechler SJ, Castell DO. Classification of oesophageal motility abnormalities. Gut. 2001;49(1):145–151. doi: 10.1136/gut.49.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pandolfino JE, Ghosh SK, Rice J, Clarke JO, Kwiatek MA, Kahrilas PJ. Classifying esophageal motility by pressure topography characteristics: a study of 400 patients and 75 controls. Am J Gastroenterol. 2008;103(1):27–37. doi: 10.1111/j.1572-0241.2007.01532.x. [DOI] [PubMed] [Google Scholar]
- 29.Behar J, Biancani P. Pathogenesis of simultaneous esophageal contractions in patients with motility disorders. Gastroenterology. 1993;105(1):111–118. doi: 10.1016/0016-5085(93)90016-6. [DOI] [PubMed] [Google Scholar]
- 30.Roman S, Lin Z, Pandolfino JE, Kahrilas PJ. Distal Contraction Latency: A Measure of Propagation Velocity Optimized for Esophageal Pressure Topography Studies. Am J Gastroenterol. 2010 doi: 10.1038/ajg.2010.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghosh SK, Pandolfino JE, Zhang Q, Jarosz A, Shah N, Kahrilas PJ. Quantifying esophageal peristalsis with high-resolution manometry: a study of 75 asymptomatic volunteers. Am J Physiol Gastrointest Liver Physiol. 2006;290(5):G988–G997. doi: 10.1152/ajpgi.00510.2005. [DOI] [PubMed] [Google Scholar]
- 32.Tutuian R, Mainie I, Agrawal A, Gideon RM, Katz PO, Castell DO. Symptom and function heterogenicity among patients with distal esophageal spasm: studies using combined impedance-manometry. Am J Gastroenterol. 2006;101(3):464–469. doi: 10.1111/j.1572-0241.2006.00408.x. [DOI] [PubMed] [Google Scholar]
- 33.Sifrim D, Janssens J, Vantrappen G. A wave of inhibition precedes primary peristaltic contractions in the human esophagus. Gastroenterology. 1992;103(3):876–882. doi: 10.1016/0016-5085(92)90020-y. [DOI] [PubMed] [Google Scholar]
- 34.Sifrim DA, Janssens JP. The 'artificial high pressure zone'. A non-invasive method to study in man the effect of the inhibitory innervation to the oesophagus. Validation study using a combined manometric--barostat technique. Eur J Gastroenterol Hepatol. 1999;11(2):165–169. doi: 10.1097/00042737-199902000-00017. [DOI] [PubMed] [Google Scholar]