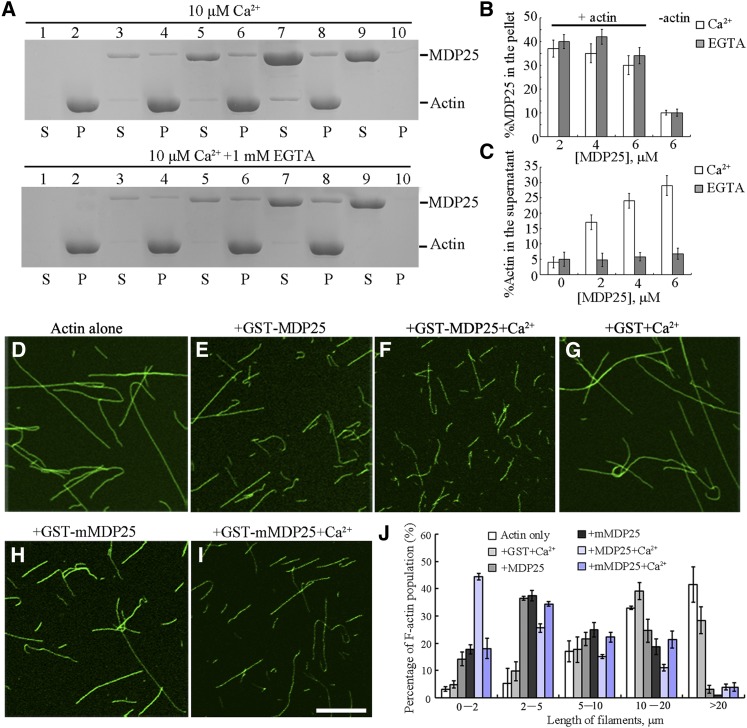
Figure 3.
MDP25 Binds and Fragments Actin Filaments in Vitro.
(A) A high-speed cosedimentation assay was used to determine F-actin binding of MDP25. SDS-PAGE assay showed GST-MDP25 protein appeared mostly in the supernatant (S) in the absence of F-actin but cosedimented with F-actin in the pellets (P) in the presence of or absence of Ca2+. Lanes 1 and 2, actin alone; lanes 3 and 4, actin in the presence of 2 μM MDP25; lanes 5 and 6, actin in the presence of 4 μM MDP25; lanes 7 and 8, actin in the presence of 6 μM MDP25; lanes 9 and 10, MDP25 alone. The top gel shows samples in the presence of 10 μM Ca2+, and the bottom gel shows the samples in the presence of 1 mM EGTA.
(B) and (C) Statistical analysis for (A).The amount of MDP25 and actin in the pellets and in the supernatant was estimated by gel density scanning and is expressed as a percentage of total MDP25 and actin, respectively. Error bars represent mean ± sd (n = 3).
(D) to (I) MDP25 fragments actin filaments. Actin filaments were visualized by the addition of 1 μM Alexa488-phalloidin. Preformed F-actin was incubated with 0 μM MDP25 (D), 1 μM GST-MDP25 (E), 1 μM GST-MDP25 plus 10 μM Ca2+ (F), 1 μM GST plus 10 μM Ca2+ (G), 1 μM GST-mMDP25 (H), and 1 μM GST-mMDP25 plus 10 μM Ca2+ (I). Bar = 10 μm.
(J) The length of F-actin was measured after incubation with or without MDP25 or mutated proteins in the presence and absence of Ca2+. Error bars represent mean ± se (n = 3).