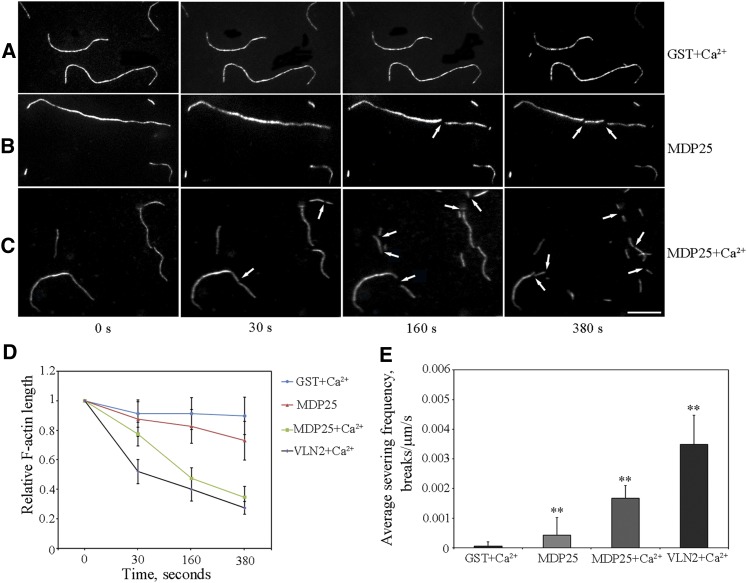
Figure 4.
MDP25 Severs Actin Filaments in Vitro.
(A) to (C) Time series of prepolymerized rhodamine-labeled actin filaments. Over a 380-s time period, actin filaments were cut into fragments after introduction of 100 nM GST-MDP25 (B) (see Supplemental Movie 2 for the entire series). More intense severing activity of MDP25 was observed when 10 μM Ca2+ was added together with MDP25 (C) (see Supplemental Movie 3 for the entire series); severing events increased and the actin filament fragments were much shorter. Actin filaments were rarely changed when 100 nM GST plus 10 μM Ca2+ was introduced (A) (see Supplemental Movie 1 for the entire series) (arrow indicates where severing occurred). Bar = 10 μm.
(D) Quantitative kinetics indicated the progressive shortening of actin filaments. More than 60 prepolymerized rhodamine-labeled actin filaments of each sample were traced in the presence of 100 nM GST plus 10 μM Ca2+, 100 nM GST-MDP25, 100 nM GST-MDP25 plus 10 μM Ca2+, or 1 nM VLN2 plus 10 μM Ca2+, respectively. Relative actin filament lengths were normalized to the initial length of the actin filaments at each time point. Data were collected from three independent experiments. Error bars indicate mean ± sd.
(E) Mean severing frequency calculated when rhodamine-actin filaments were incubated with 100 nM GST plus 10 μM Ca2+ or 100 nM GST-MDP25 and 100 nM GST-MDP25 plus 10 μM Ca2+, respectively. A mean severing frequency of 1 nM VLN2 plus 10 μM Ca2+ was used as a positive control. Experiments were repeated three times. Each experiment examined more than 18 filaments. Results are presented as mean ± se. **P < 0.01 compared with 100 nM GST plus Ca2+ by the Student’s t test.