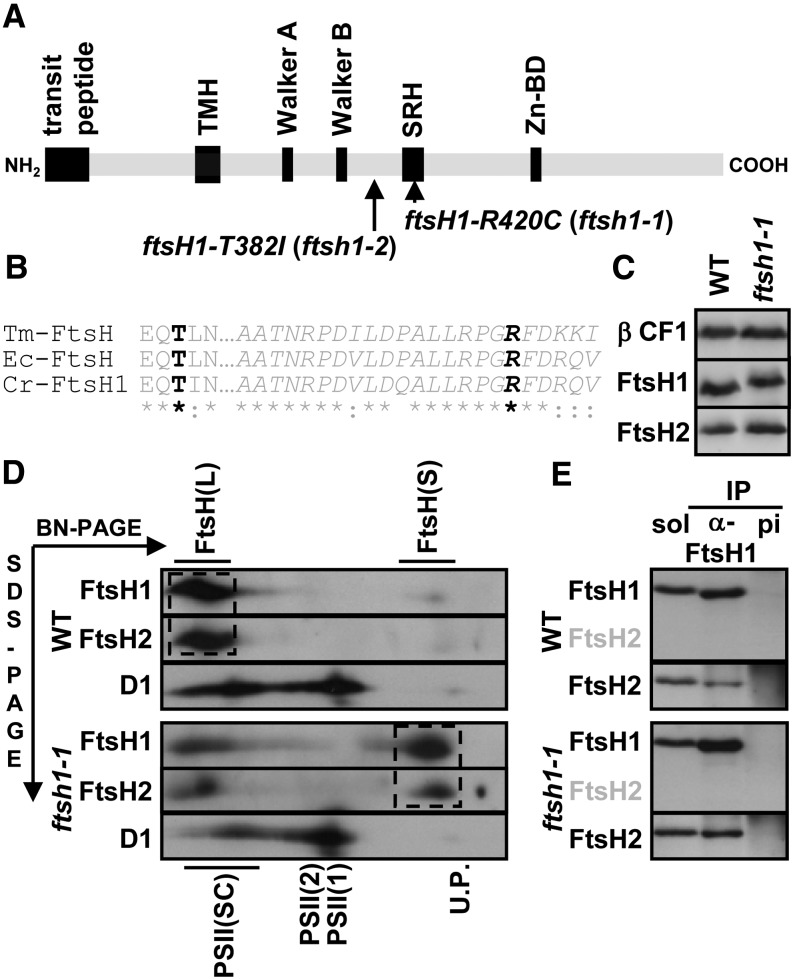
Figure 3.
Mutant ftsH1-R420C Accumulates Wild-Type Levels of FtsH1 and FtsH2 but with Impaired Oligomerization.
(A) Schematic representation of ATP-dependent zinc metalloprotease FtsH1 with the transit peptide, one thylakoid transmembrane helix (TMH), ATPase Walker A and B motifs, the SRH containing conserved Arg residues required for ATP hydrolysis (one of which is substituted to a Cys residue in the ftsH1-R420C [ftsh1-1] mutant of C. reinhardtii), and the zinc binding domain (Zn-BD).
(B) Alignment of the SRH (in italics) from T. maritima FtsH (Tm-FtsH), E. coli FtsH (Ec-FtsH), and C. reinhardtii FtsH1 (Cr-FtsH1), indicating the conservation of the Arg and Thr residues (in black) substituted in the ftsh1-1 and ftsh1-2 mutants.
(C) Mutant ftsH1-R420C accumulates wild-type levels of FtsH1 and FtsH2. Total cell proteins were separated on an 8% polyacrylamide gel in the presence of 8 M urea and analyzed by immunodetection with specific antibodies against FtsH1, FtsH2, and the ATP synthase β-subunit (β CF1) as a loading control.
(D) FtsH complexes after BN-PAGE of digitonin-solubilized membranes from cells grown at 6 μE m−2 s−1. Digitonin-solubilized proteins were separated in the first dimension by BN-PAGE (3 to 12%) and in the second dimension as in (C) and blotted onto a polyvinylidene difluoride membrane. The blot was sequentially probed with antibodies specific for FtsH2, FtsH1, and D1. FtsH1 and FtsH2 migrated mainly and similarly in two populations, the smaller FtsH(S) and the larger FtsH(L) proteins. FtsH(L) is abundant in the wild type and FtsH(S) in ftsh1-1 (dashed boxes). The positions of unassembled proteins (U.P.), monomers [PSII(1)], dimers [PSII(2)], and supercomplexes [PSII(SC)] of PSII are indicated and used as molecular mass markers.
(E) Coimmunoprecipitation analysis of FtsH1 and FtsH2 interactions. Digitonin-solubilized membranes (prepared as for BN-PAGE) from the wild-type and ftsh1-1 strains were incubated with protein A–Sepharose beads coupled to antibodies against a peptide of FtsH1 (α-FtsH1) or against preimmune serum (pi). Aliquots of digitonin-solubilized membranes (sol) and immunoprecipitates (IP) were separated as in (C) and analyzed by immunodetection with antibodies against FtsH1 (not cross-reacting with FtsH2, as indicated with gray letters) and FtsH2.