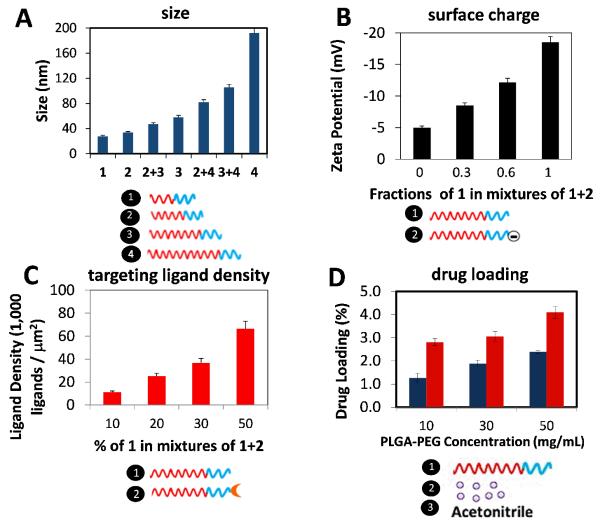
Figure 2. Synthesis of PLGA-PEG NPs with control over physicochemical properties.
(A) Variation of NP size by mixing polymers with different PLGA MW. 1 – PLGA10K-PEG5K; 2 – PLGA27K-PEG5K; 3 – PLGA45K-PEG5K; 4 – PLGA95K-PEG5K. (B) Variation of NP surface charge by mixing polymers with different end functional group on the PEG block. Carboxyl group ( – COOH) is negatively charged while methoxy group (-OCH3) is neutral. 1 – PLGA45K-PEG5KOCH3; 2 – PLGA45K-PEG5K-COOH. (C) Variation of targeting ligand density by mixing polymers functionalized with targeting ligand (PLGA-PEG-LIG) together with unmodified polymer. 1 – PLGA27K-PEG5K; 2 – PLGA45K-PEG5K-LIG. (D). Variation of drug loading by mixing a polymer at a high concentration (50 mg/mL) with different ratios of drug (to vary initial drug loading) and plain acetonitrile (to vary concentration). 1 – PLGA27K-PEG5K; 2 – docetaxel; 3 – acetonitrile. Blue bar, initial drug loading of 5%; red bar, initial drug loading of 10%. Legend: Blue block = PEG; Red block = PLGA. Error bars denote ±S.D.