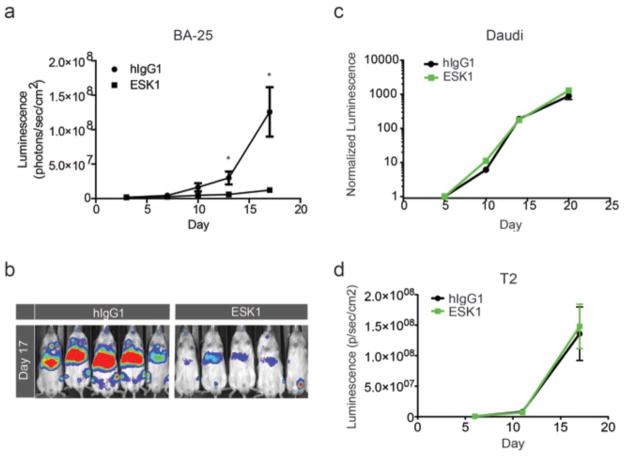
Fig. 6. ESK1 specifically treats a second A2+ leukemia, but not A2− or WT1-tumors.
(a–b) Three million BA25 human acute lymphocytic leukemia cells (luciferase positive) were injected IV into NSG mice. On day 3, tumor engraftment was confirmed by firefly luciferase imaging in all mice that were to be treated; mice were then randomly divided into different treatment groups. On day 4 and day 8, 100ug mAb ESK1 or the isotype control mAb were injected IV. Tumor growth was assessed by luminescence imaging once to twice a week, and clinical measurements were assessed daily. Significant reductions in the growth of the ALL cells by the ESK1 mAb was seen relative to animals given the isotype control (a) by quantitation of luminescence from the supine animals (p < 0.04 and p < 0.02 on days 13 and 17, respectively.) These data are represented in a day 17 image (b). Treatment with 100ug mAb on days 6 and 10 after injection of 5 million Daudi ALL3. Leukemia cells (c) or 2 million T2 leukemia cells was followed and quantitated by bioluminescent imaging.