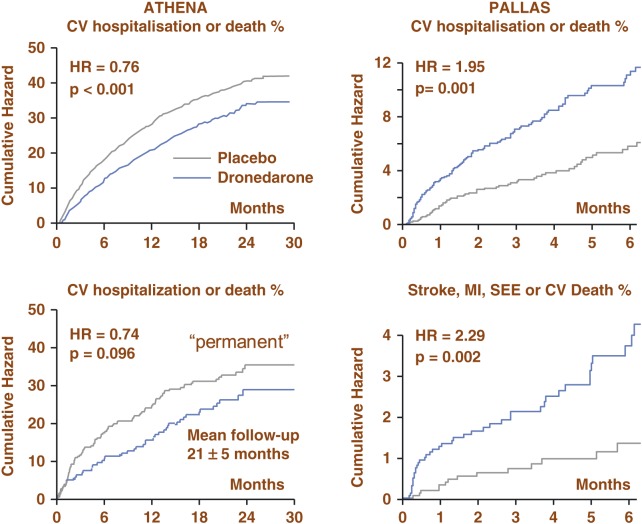
Figure 4. .
Summary of outcomes in PALLAS and ATHENA trials. In PALLAS, there was an increased incidence in the first coprimary outcome of stroke, myocardial infarction, systemic embolism or CV death for dronedarone compared to placebo and an increased incidence in the second coprimary outcome unplanned cardiovascular hospitalisation or death for dronedarone compared to placebo. As a result dronedarone should not be used in this permanent high risk AF patient population. Standard therapy may have included rate control agents (beta-blockers, and/or Ca-antagonist and/or digoxin) and/or anti-thrombotic therapy (Vit. K antagonists and /or aspirin and other antiplatelets therapy) and/or other cardiovascular agents such as ACEIs/ARBs and statins. Source: Adapted from Hohnloser et al. 45 ; Connolly et al. 46