Figure 4. .
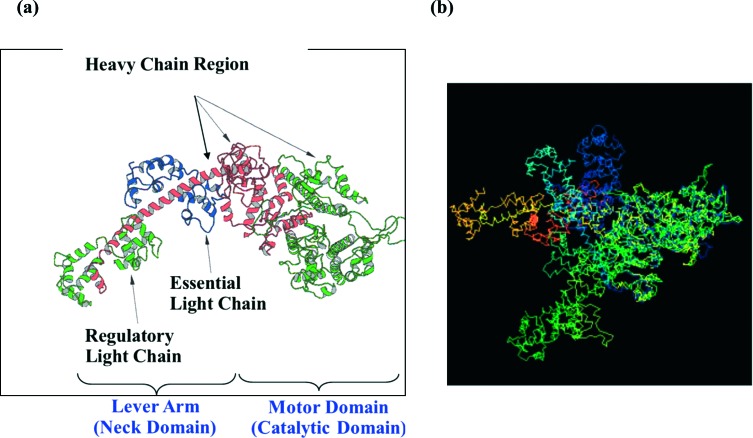
(a) Structure of the myosin head in the rigor state with no nucleotide bound solved by X-ray crystallography (4). The head consists of a single heavy chain and two light chains, named the essential light chain (ELC) and the regulatory light chain (RLC). It has a motor domain and a neck domain or lever arm. The motor domain is the catalytic domain which has an ATP-binding site and the actin-binding site. The lever arm is a single α-helical with the two light chains (ELC and RLC) bound to it and it connects to the rod portion of the myosin molecules forming the backbone. There are two heads connected to the rod which form a myosin molecule (shown Figure 3a). (b) Comparison of the myosin head structure from various published crystal structures with different nucleotides bound. All the structures are superimposed at the catalytic motor domain, so that the differences between the models are expressed by the different positions of their lever arms. Green and pointing slightly towards the viewer is the 4 chicken skeletal myosin with no nucleotide bound (i.e. rigor-like); dark blue is the 5 chicken smooth muscle myosin in ADP.AlF4 form; pale blue is the Lethocerus structure in the relaxed state (6); orange is the 7 scallop myosin in Mg.ADP.VO4 form. The view is in the direction such that the actin filament axis is vertical and to the right of the myosin head, with the M-band at the top and Z-band at the bottom.