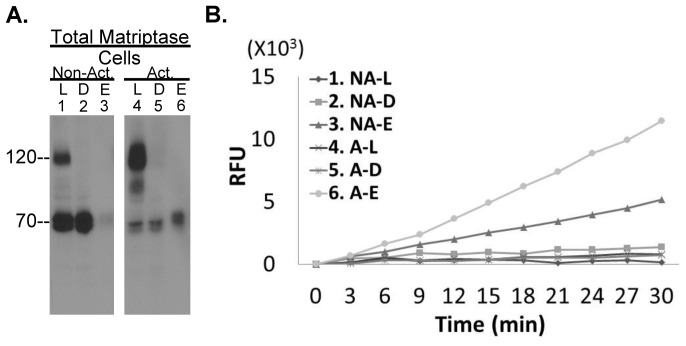
Figure 1. MCF breast cancer cells don't retain enzymatically active matriptase.
A. MCF7 human breast cancer cells were incubated with a pH 6 buffer to induce matriptase zymogen activation (A. right, Act.) or with the pH 6 buffer supplemented with 150 mM NaCl as a non-activation control (A. left, Non-act.). Cell lysates were prepared, and samples retained for analysis (lanes 1 and 4). The remaining cell lysates were subjected to immunodepletion with the active matriptase-specific mAb M69 immobilized on Sepharose beads to deplete the activated matriptase, predominantly the 120-kDa complex (lanes 2 and 5, and D). The antibody-bound activated matriptase was recovered by a pH 2.4 buffer elution followed by pH neutralization (Lane 3, E). These samples were then analyzed for matriptase species by SDS-PAGE (without boiling the samples or using reducing agents) and Western blot using the total matriptase mAb M24. B. The cells, the immunodepleted lysates, and the eluates were assayed tryptic activity by cleavage of a synthetic fluorogenic substrate with Arg as P1 site. For the tryptic assay, the cells remained intact in the absence of Triton X-100. NA stands for non-activation; L for loading of immunodepletion, D for immunodepleted fraction; E for eluted fraction; A for activation; RFU for relative fluorescent unit.