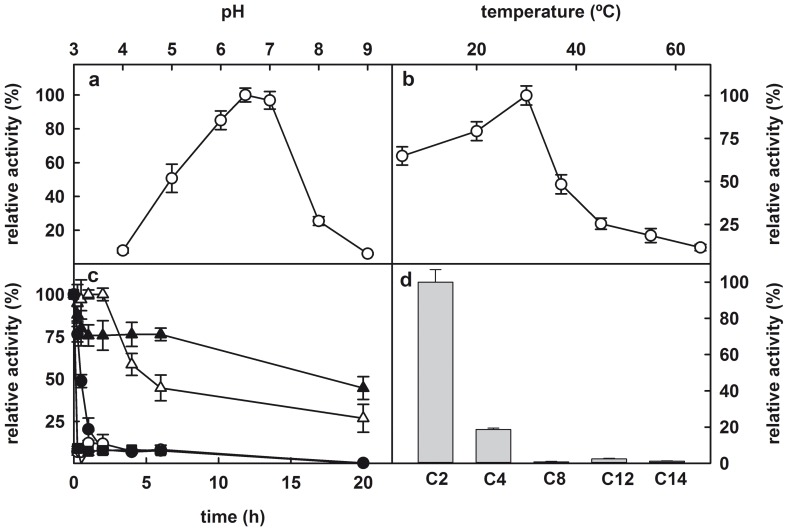
Figure 11. Biochemical characterization of LpEst1.
(A) Dependence on pH of hydrolytic activity of LpEst1 against pNPA. (B) Dependence on temperature of hydrolytic activity of LpEst1 against pNPA. The optimum temperature for esterase activity was ∼30 °C. (C) Analysis of the temperature stability of LpEst1. The enzyme was incubated in 50 mM sodium phosphate buffer pH 7.0 at 22 °C (closed triangles), 30 °C (open triangles), 37 °C (closed circles), 45 °C (open circles) and 55 °C (closed squares) for 15 min, 30 min, and 1, 2, 4, 6 and 20 h. The values correspond to the mean of three independent experiments. (D) Dependence of the esterase activity of LpEst1 on the chain length of p-nitrophenyl (p-NP): p-NP acetate (C2), p-NP butyrate (C4); p-NP caprylate (C8); p-NP laureate (C12); and p-NP myristate (C14).