Figure 2. Chemoreceptor arrays.
A. Proposed chemoreceptor array model.
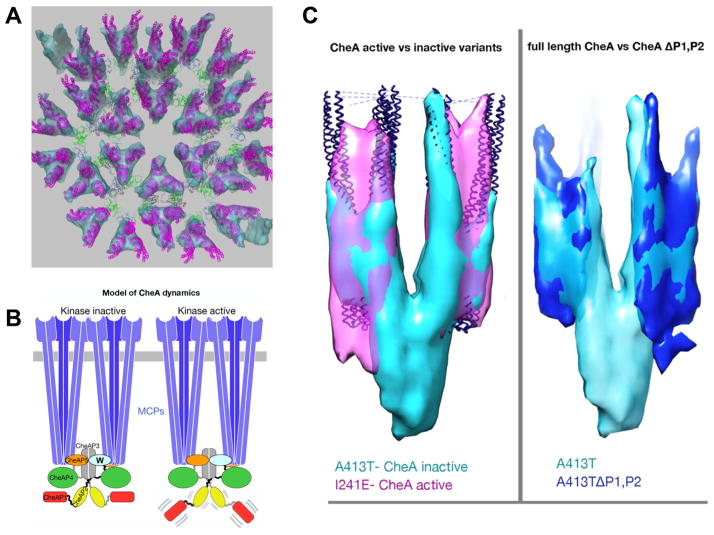
This model is based on the crystal structure 3UR1 and the EM map of wild-type E. coli arrays (Briegel et al., 2012). Receptor trimer-of-dimers, purple; CheW, green; CheA-P5, blue; CheA-P4, dark grey; and CheA-P3, light grey.
B. Proposed model for signaling through mobility control of the kinase domains P1 AND P2. In a kinase-off signaling state, the CheA domain P1 is trapped in a static, nonproductive interaction with P4, constraining the P1 and P2 domains to the keel volume. In the kinase-on signaling state, increased mobility of the P1 and P2 domains promotes cycles of P1 engagement, phosphorylation and release.
C. The mobility of CheA P1 and P2 domains differs between kinase activity states. Left:Overlay of chemoreceptor Tsr variants locked in the CheA inactivating state (A413T, turquoise) and CheA activating state (I241E, magenta). For reference and alignment, receptor crystal structures (blue) were fitted into the sub-tomogram averages by molecular-dynamics-based-flexible-fitting (MDFF). A prominent ‘keel density’ is visible in the kinase inactive state underneath the chemoreceptors, but absent in the kinase active state indicating increased mobility of the keel forming domains. Right: Overlay of Tsr A413T variants with full length CheA (turquoise) and CheA lacking the P1 and P2 domains (blue) reveals CheA domains P1 and P2 as the major components of the keel.