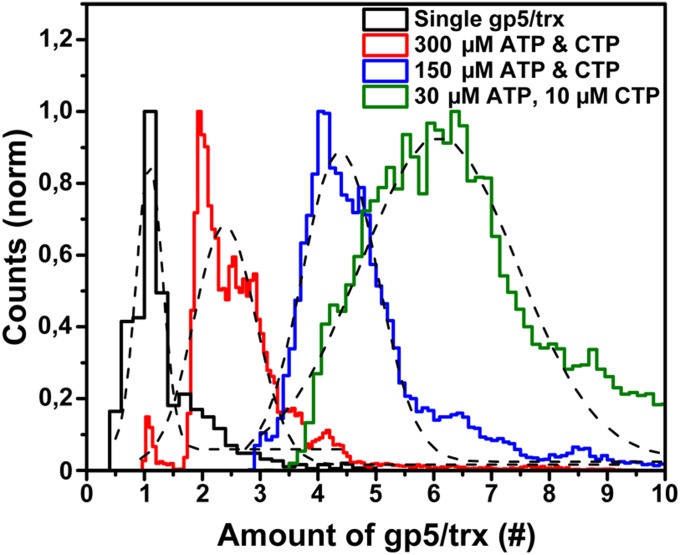
Fig. 4.
Stoichiometry of polymerases at the replication fork. The steady-state binding of polymerases at the replisome for different ATP and CTP concentrations was determined by dividing the fluorescence intensity at the replisome, averaged over 10 s by a sliding average window, by the intensity of a single labeled polymerase. The total fluorescence represents the summation of the number of Alexa Fluor 488- and Cy5-labeled polymerases. Fitting the distributions with Gaussians (black) resulted in maximum values with a SD of 2.4 ± 0.3 gp5/trx, 4.4 ± 0.3 gp5/trx, and 6.1 ± 0.7 gp5/trx for 300 µM ATP and CTP, 150 µM ATP and CTP, and 30 µM ATP with 10 µM CTP, respectively.