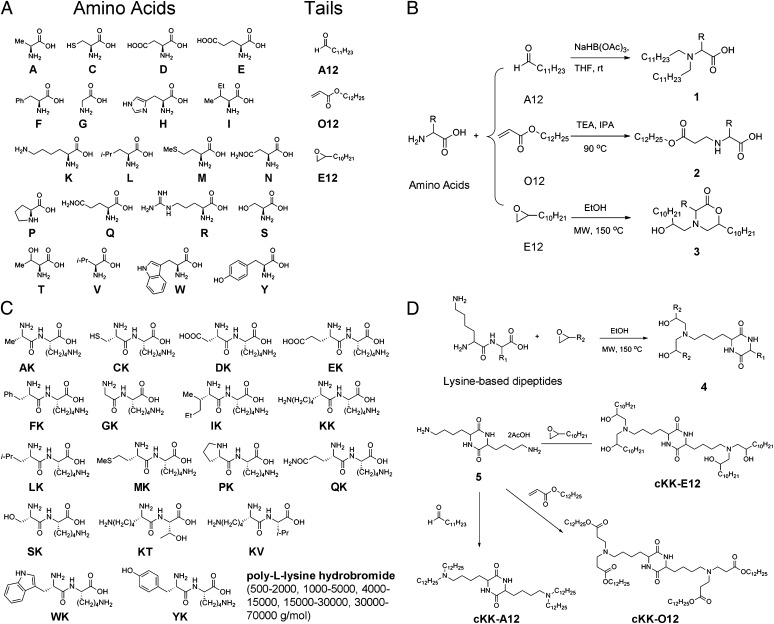
Fig. 2.
Synthetic routes to 103 lipoamino acid, lipopeptide, and lipopolypeptide derivatives. (A) A list of amino acids and lipid tails as starting materials. (B) Single amino acids were reacted with aldehydes, acrylates, and epoxides to install lipid tails and produce 1–3. (C) Lysine-based dipeptides and polypeptides include KG, KT, YK, LK, DK, MK, KV, AK, CK, QK, PK, FK, WK, EK, IK, SK, and poly-l-lysine hydrobromide [molecular weight (g/mol): 500–2,000 (PK500), 1,000–5,000 (PK1K), 4,000–15,000 (PK4K), 15,000–30,000 (PK15K), and 30,000–70,000 (PK30K)]. (D) Microwave irradiation of epoxides and dipeptides dramatically reduced the reaction time from 3 d to 5 h. Reactions between lysine–lysine and poly-l-lysine and aldehydes and acrylates were similar to those of single amino acids in Fig. 2B. Diamine 5 was reacted with 1,2-epoxydodecane, dodecanal, or dodecyl acrylate to afford cKK-E12, cKK-A12, and cKK-O12.