Abstract
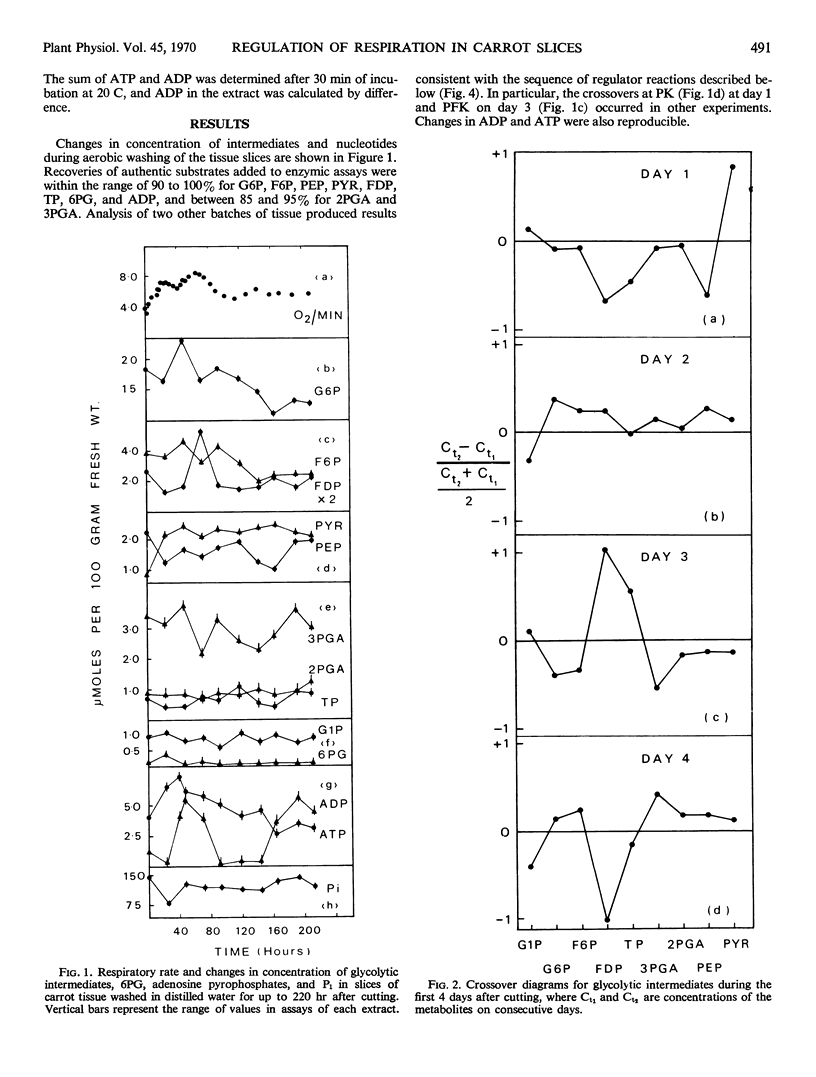
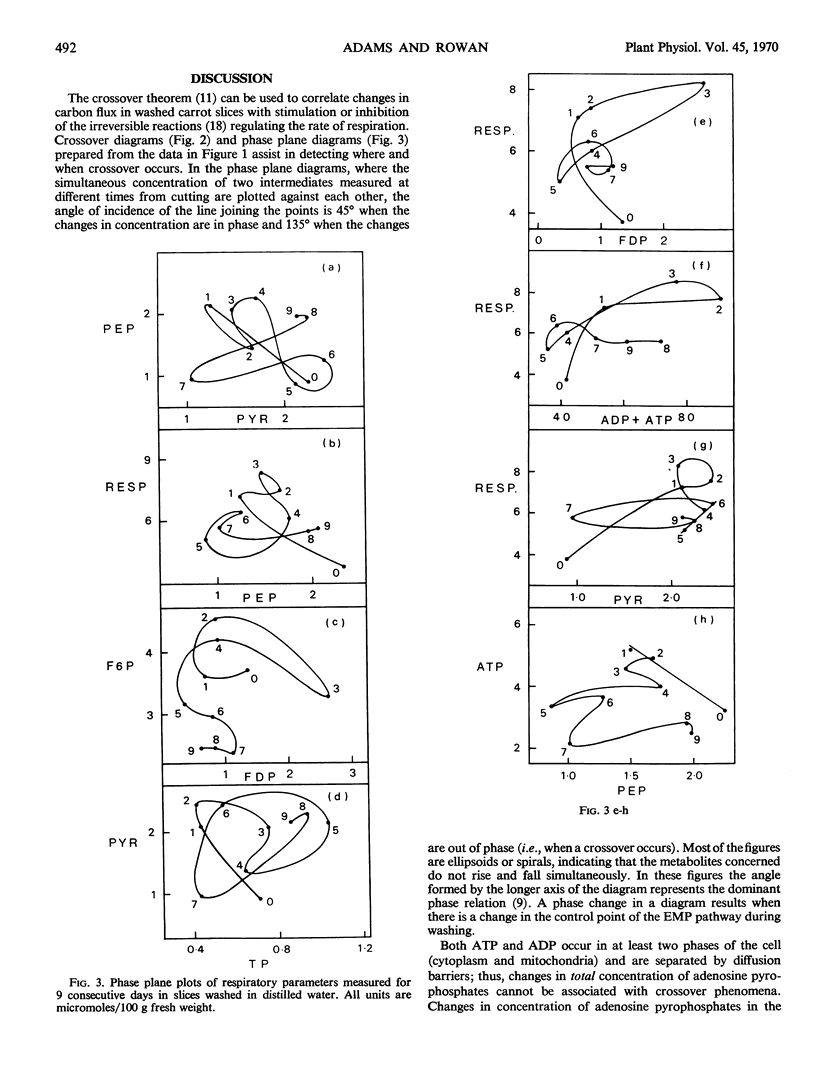
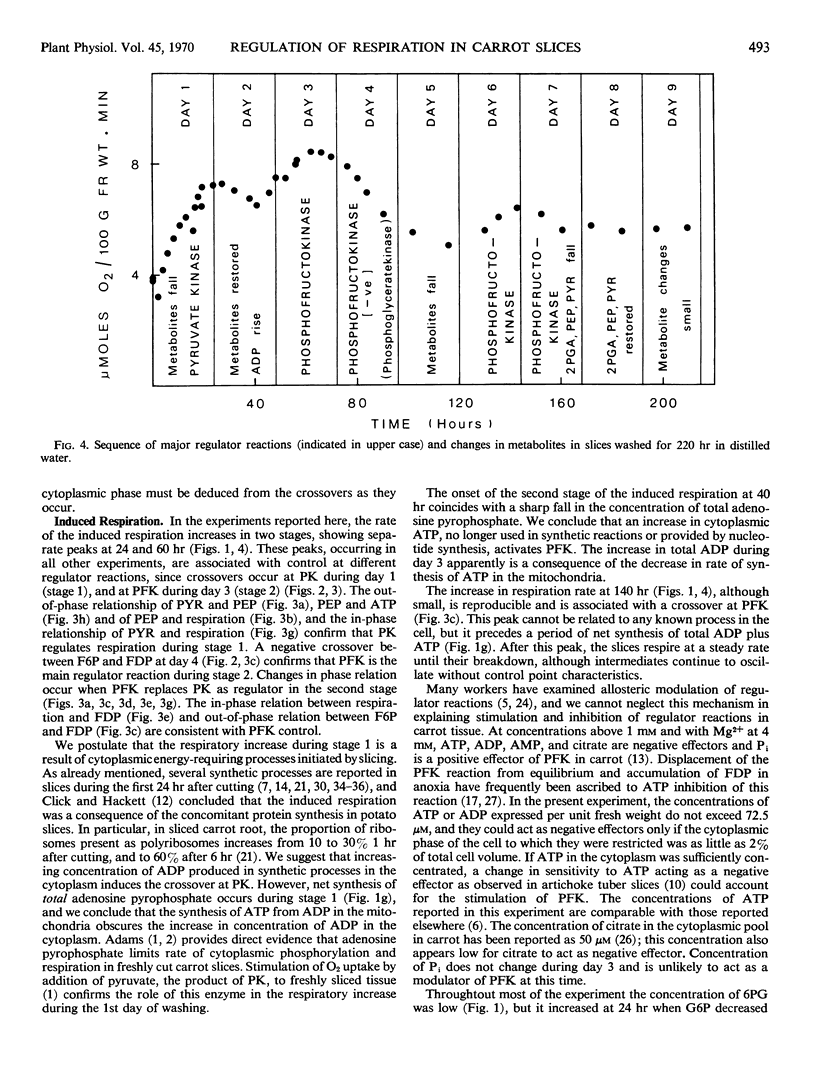
Changes in respiration in aging carrot slices are correlated with a characteristic sequence of activation of the irreversible reactions of the glycolytic pathway. The induced “wound” respiration increases in two stages; these appear to correspond to a period of active synthesis, when ADP, produced in the cytoplasm from synthetic reactions, activates pyruvate kinase, and a period of readjustment when ATP, no longer reacting in synthetic reactions, activates phosphofructokinase. Although ATP is cited frequently as a negative effector of phosphofructokinase, unless the concentration of ATP at the site of phosphofructokinase is 50-fold higher than the concentration expressed per unit fresh weight of the tissue, it cannot act in this way in carrot slices. The activation of the oxidative pentose phosphate pathway observed by ap Rees and Beevers is restricted to the first stage of the induced respiration.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. B. Effect of adenine nucleotides on levels of glycolytic intermediates during the development of induced respiration in carrot root slices. Plant Physiol. 1970 Apr;45(4):500–503. doi: 10.1104/pp.45.4.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. B. Effect of adenine nucleotides on the respiration of carrot root slices. Plant Physiol. 1970 Apr;45(4):495–499. doi: 10.1104/pp.45.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R. J. The estimation of phosphorus. Biochem J. 1940 Jun;34(6):858–865. doi: 10.1042/bj0340858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson D. E. Biological feedback control at the molecular level. Science. 1965 Nov 12;150(3698):851–857. doi: 10.1126/science.150.3698.851. [DOI] [PubMed] [Google Scholar]
- BACON J. S., MACDONALD I. R., KNIGHT A. H. THE DEVELOPMENT OF INVERTASE ACTIVITY IN SLICES OF THE ROOT OF BETA VULGARIS L. WASHED UNDER ASEPTIC CONDITIONS. Biochem J. 1965 Jan;94:175–182. doi: 10.1042/bj0940175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BETZ A., CHANCE B. PHASE RELATIONSHIP OF GLYCOLYTIC INTERMEDIATES IN YEAST CELLS WITH OSCILLATORY METABOLIC CONTROL. Arch Biochem Biophys. 1965 Mar;109:585–594. doi: 10.1016/0003-9861(65)90404-2. [DOI] [PubMed] [Google Scholar]
- Black M. K., Wedding R. T. Effects of storage and aging on properties of phosphofructokinase from jerusalem artichoke tubers. Plant Physiol. 1968 Dec;43(12):2066–2069. doi: 10.1104/pp.43.12.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., HOLMES W., HIGGINS J., CONNELLY C. M. Localization of interaction sites in multi-component transfer systems: theorems derived from analogues. Nature. 1958 Nov 1;182(4644):1190–1193. doi: 10.1038/1821190a0. [DOI] [PubMed] [Google Scholar]
- CLICK R. E., HACKETT D. P. THE ROLE OF PROTEIN AND NUCLEIC ACID SYNTHESIS IN THE DEVELOPMENT OF RESPIRATION IN POTATO TUBER SLICES. Proc Natl Acad Sci U S A. 1963 Aug;50:243–250. doi: 10.1073/pnas.50.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis D. T., Coultate T. P. Phosphofructokinase, a regulatory enzyme in plants. Biochem Biophys Res Commun. 1966 Oct 20;25(2):187–191. doi: 10.1016/0006-291x(66)90578-x. [DOI] [PubMed] [Google Scholar]
- EDELMAN J., HALL M. A. ENZYME FORMATION IN HIGHER-PLANT TISSUES. DEVELOPMENT OF INVERTASE AND ASCORBATE-OXIDASE ACTIVITIES IN MATURE STORAGE TISSUE OF HELIANTHUS TUBEROSUS L. Biochem J. 1965 May;95:403–410. doi: 10.1042/bj0950403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson R. G., Rowan K. S. Phosphate Metabolism and Induced Respiration in Washed Carrot Slices. Plant Physiol. 1965 Nov;40(6):1247–1250. doi: 10.1104/pp.40.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A., Chance B. Oscillations of glycolytic intermediates in yeast cells. Biochem Biophys Res Commun. 1964 Jun 1;16(2):174–181. doi: 10.1016/0006-291x(64)90357-2. [DOI] [PubMed] [Google Scholar]
- HOMMES F. A. OSCILLATORY REDUCTIONS OF PYRIDINE NUCLEOTIDES DURING ANAEROBIC GLYCOLYSIS IN BREWERS' YEAST. Arch Biochem Biophys. 1964 Oct;108:36–46. doi: 10.1016/0003-9861(64)90352-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V. THE RELATIONSHIPS BETWEEN SUBSTRATES AND ENZYMES OF GLYCOLYSIS IN BRAIN. J Biol Chem. 1964 Jan;239:31–42. [PubMed] [Google Scholar]
- Leaver C. J., Key J. L. Polyribosome formation and RNA synthesis during aging of carrot-root tissue. Proc Natl Acad Sci U S A. 1967 May;57(5):1338–1344. doi: 10.1073/pnas.57.5.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou M. F., Kinoshita J. H. Control of lens glycolysis. Biochim Biophys Acta. 1967 Aug 29;141(3):547–559. doi: 10.1016/0304-4165(67)90184-5. [DOI] [PubMed] [Google Scholar]
- Maclennan D. H., Beevers H., Harley J. L. 'Compartmentation' of acids in plant tissues. Biochem J. 1963 Nov;89(2):316–327. doi: 10.1042/bj0890316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakami S. Studies on leukocyte metabolism. I. J Biochem. 1968 Jan;63(1):83–88. doi: 10.1093/oxfordjournals.jbchem.a128752. [DOI] [PubMed] [Google Scholar]
- Rees T. A., Beevers H. Pentose Phosphate Pathway as a Major Component of Induced Respiration of Carrot and Potato Slices. Plant Physiol. 1960 Nov;35(6):839–847. doi: 10.1104/pp.35.6.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose I. A., Warms J. V. Control of glycolysis in the human red blood cell. J Biol Chem. 1966 Nov 10;241(21):4848–4854. [PubMed] [Google Scholar]
- Steward F. C., Preston G. METABOLIC PROCESSES OF POTATO DISCS UNDER CONDITIONS CONDUCIVE TO SALT ACCUMULATION. Plant Physiol. 1940 Jan;15(1):23–61. doi: 10.1104/pp.15.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W. J., Castelfranco P. A. Phospholipid synthesis in aging potato tuber tissue. Plant Physiol. 1968 Aug;43(8):1232–1238. doi: 10.1104/pp.43.8.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood A. H., Newsholme E. A. Control of glycolysis and gluconeogenesis in rat kidney cortex slices. Biochem J. 1967 Jul;104(1):300–305. doi: 10.1042/bj1040300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan D., Macdonald I. R. Development of soluble and insoluble invertase activity in washed storage tissue slices. Plant Physiol. 1967 Mar;42(3):456–458. doi: 10.1104/pp.42.3.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verleur J. D., Uritani I. Respiratory Activity of the Mitochondrial Fractions Isolated from Healthy Potato Tubers and from Tuber Tissue Incubated after Cutting or Infection with Ceratocystis fimbriata. Plant Physiol. 1965 Nov;40(6):1008–1012. doi: 10.1104/pp.40.6.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU R. CONTROL MECHANISMS OF GLYCOLYSIS IN EHRLICH ASCITES TUMOR CELLS. J Biol Chem. 1965 Jul;240:2827–2832. [PubMed] [Google Scholar]
- WU R. RATE-LIMITING FACTORS IN GLYCOLYSIS AND INORGANIC ORTHOPHOSPHATE TRANSPORT IN RAT LIVER AND KIDNEY SLICES. J Biol Chem. 1965 Jun;240:2373–2381. [PubMed] [Google Scholar]


