Fig. 5. DEXA- and mCT-derived measurements of bone mass, density, and architecture in female Lrp5+/+ and Lrp5−/− mice that had been treated for 3 weeks with vehicle or sclerostin antibody (Scl-AbIII).
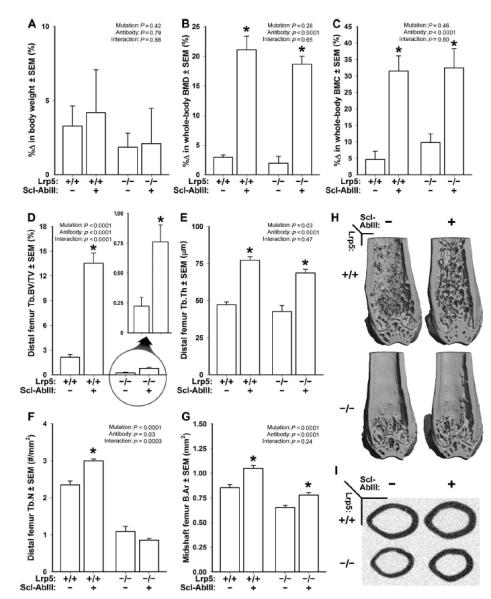
(A) Percent change in body weight over the 3-week experimental period. (B) Percent change in whole-body aBMD over the 3-week experimental period. (C) Percent change in whole-body BMC over the 3-week experimental period. (D) Distal femur trabecular bone volume fraction (BV/TV) after 3 weeks of treatment with vehicle or antibody. An enlarged view of the antibody effect in Lrp5−/− mice is provided (circle with arrow) because baseline BV/TV is so low in these mice. (E) Trabecular thickness (Tb.Th) after 3 weeks of treatment with vehicle or antibody. (F) Trabecular number (Tb.N) after 3 weeks of treatment with vehicle or antibody. (G) Midshaft femur cortical bone area (B.Ar) after 3 weeks of treatment with vehicle or antibody. (H) Representative cut-away (anterior portion digitally removed to reveal the metaphysis) mCT images of distal femur from the treatment groups indicated in (A) to (G). (I) Representative midshaft femur mCT slice from the treatment groups indicated in (A) to (G). The data were analyzed by two-way ANOVA using Lrp5 genotype and antibody/vehicle treatment as main effects (indicated at the top of each data panel). Post hoc tests comparing antibody treatment to vehicle treatment within Lrp5 genotypes were conducted using Fisher’s PLSD. *P < 0.05, significant difference from vehicle-treated mice. For each group, n = 8 mice.
