Fig. 6. Midshaft femur fluorochrome-derived BFRs on the periosteal surface of 20-week-old female Lrp5+/+ and Lrp5−/− mice that had been treated for 3 weeks with vehicle or a sclerostin antibody (Scl-AbIII).
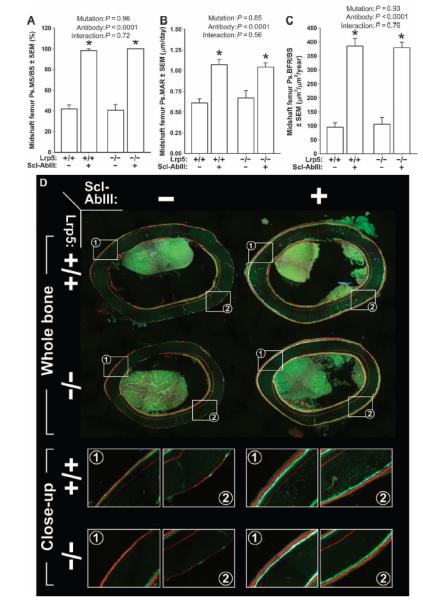
(A) Periosteal MS/BS (Ps.MS/BS). (B) Periosteal MAR (Ps.MAR). (C) Periosteal BFR per unit bone surface (Ps.BFR/BS). All three indices were derived using a calcein label given at 18 weeks of age [green label in (D)] and an alizarin complexone label given at 19 weeks of age [red label in (D)]. (D) Whole-bone (upper panels) and close-up (lower panels; taken from the white boxes indicated in the upper panels) photomicrographs of representative midshaft femur sections from each of the groups studied. A xylenol orange label can be seen in some of the sections buried deeper in the cortex (given at 12 weeks of age), which served as a pretreatment marker and was not used for any of the dynamic measurements. The data were analyzed by two-way ANOVA using Lrp5 genotype and antibody/vehicle treatment as main effects (indicated at the top of each panel). Post hoc tests comparing antibody treatment to vehicle treatment within Lrp5 genotypes were conducted using Fisher’s PLSD. *P < 0.05, significant difference from vehicle-treated mice. For each group, n = 8 mice.
