Table 1.
Scope of the coupling of alkyl- and arylboronic acids to 1,4-benzoquinone.a
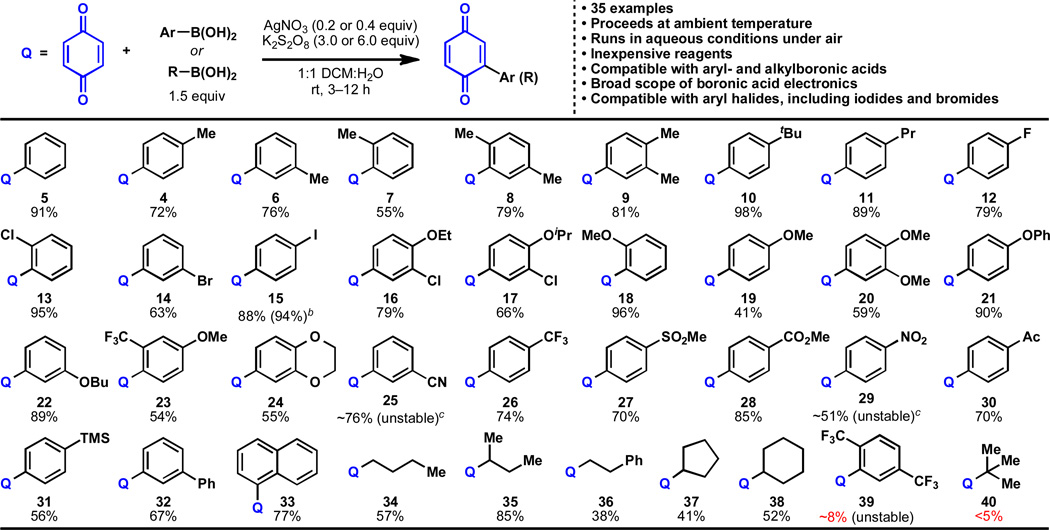 |
Benzoquinone (0.25 mmol), boronic acid (0.375 mmol), AgNO3 (0.05 mmol), K2S2O8 (0.75 mmol), 23 °C, 3–12 h; isolated yields of chromatographically and spectroscopically pure products yields displayed, unless otherwise noted. (NH4)2S2O8 is also suitable as an oxidant.
Yield of the reaction performed on gram-scale with no organic solvent (see Figure 2).
Products were unstable to several isolation conditions, but proved relatively stable in solution after extraction. See Supporting Information for more details.
