Table 1.
Substrate Scope for the Dienone-Photorearrangement-Cycloadditiona
| Entry | Substrate | Major Product | Yield [%]b, c | Entry | Substrate | Major Product | Yield [%]b,c |
|---|---|---|---|---|---|---|---|
| 1 | 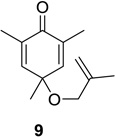 |
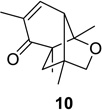 |
83 (10:1) | 7 | 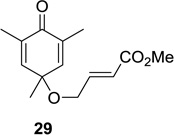 |
 |
80 (>25:1) |
| 2 | 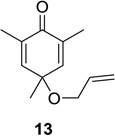 |
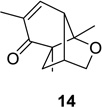 |
80 (9:1) | 8 | 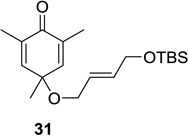 |
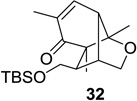 |
53 (12:1) |
| 3 | 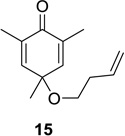 |
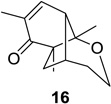 |
85 (>25:1) | 9 | 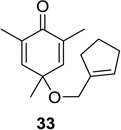 |
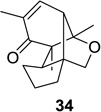 |
67 (4:1) |
| 4 | 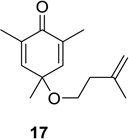 |
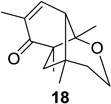 |
78 (>25:1) | 10 |  |
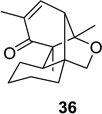 |
73 (4:1) |
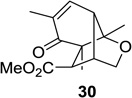
| 5 | 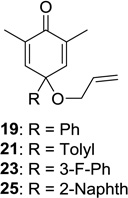 |
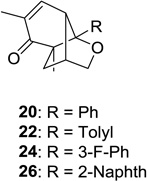 |
20: 75 (10:1) 22: 63 (4:1)d 24: 60 (4:1)d 26: 60 (4:1)d |
11 | 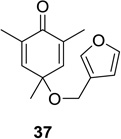 |
 |
51 (7:1) |
| 6 | 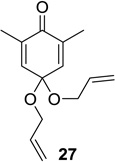 |
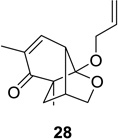 |
52 (9:1) | 12 | 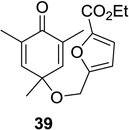 |
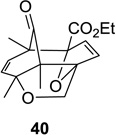 |
81 (>25:1) |
Reaction conditions: substrate (0.25 mmol) in benzene (10 mL) degassed for 30 min followed by irradiation (>300 nm) at room temperature for 20 min.
Isolated yield.
Regioselectivity determined by 1H NMR analysis of the crude reaction mixture in parenthesis.
Isolated yield of the 5,1-product.
