FIGURE 4.
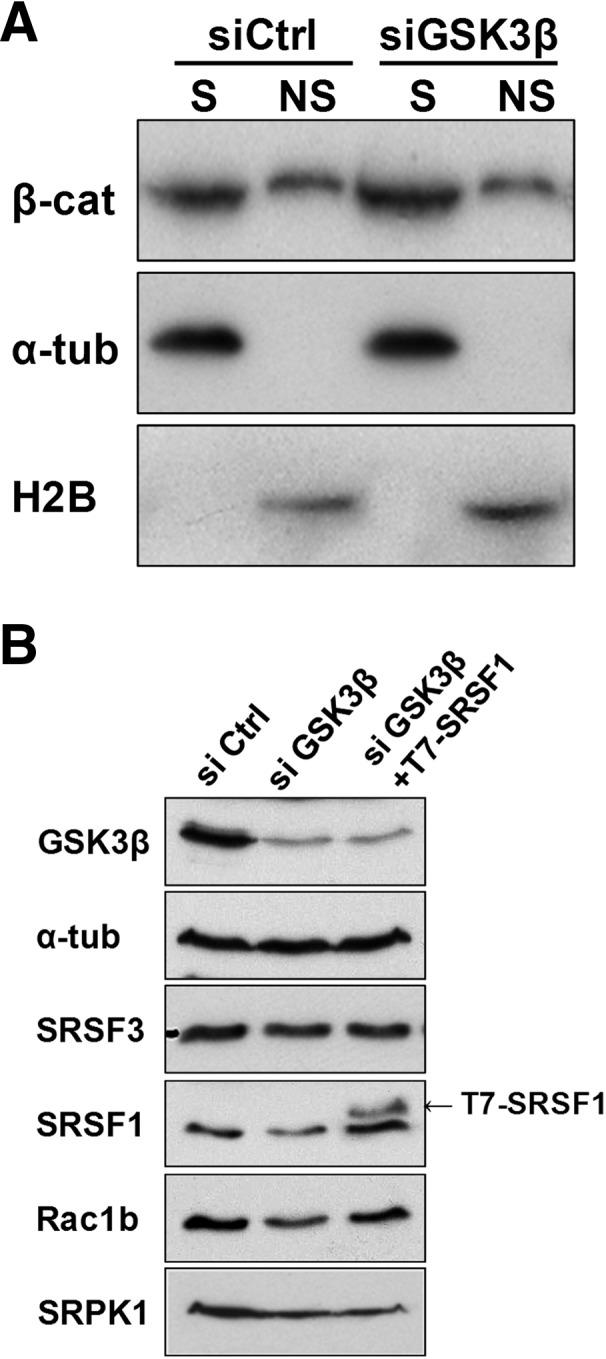
Analysis of the mechanism mediating the effect of GSK3β on Rac1b. (A) HT29 colorectal cells were transfected with the indicated siRNA oligonucleotide and nuclear chromatin-associated β-catenin was detected 48 h after transfection by Western blot. A detergent-based cell fractionation methodology separated β-catenin into a soluble (S) and a nonsoluble, chromatin-bound (NS) pool. Histone2B and α-tubulin protein levels were probed as controls for NS and S fractions, respectively. (B) Effect of GSK3β depletion on endogenous SRSF3, SRSF1, Rac1b, and SRPK1 protein levels in HT29 cells. Note that the depletion led to decreased levels of SRPK1, SRSF1, and Rac1b but had no effect on SRSF3. The third lane corresponds to cotransfection with a T7-SRSF1 expression vector 24 h after GSK3β depletion. Detection of GSK3β and α-tubulin served as controls.
