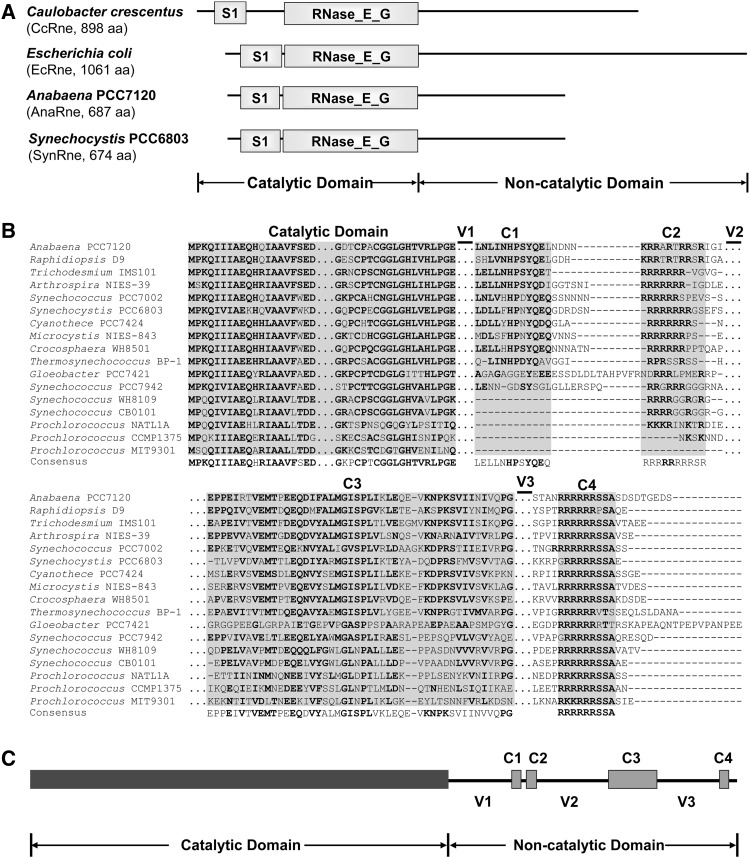
FIGURE 1.
(A) The domain structures of the RNase E proteins from Caulobacter crescentus, Escherichia coli, Anabaena PCC7120, and Synechocystis PCC6803. The catalytic domain of each RNase E is composed of a S1 domain and an RNase_E_G domain. The C-terminal noncatalytic domains of the two cyanobacterial strains show no similarity to those of other bacteria including the two proteobacterial strains listed here. (B) Alignment of RNase E proteins from 17 representative cyanobacterial strains. The alignment was performed using MEGA5 (Tamura et al. 2011) and refined manually. Four short conserved subregions (C1–C4) and three variable subregions (V1–V3) were revealed in the noncatalytic domains. The regions not shown are depicted with triple dots (…). Gaps in the alignment are depicted with short horizontal lines (-). The residues with high conservation are in bold. (C) Schematic structure of the Anabaena RNase E based on the alignment shown above. The variable and conserved regions of AnaRne revealed by the sequence alignment are depicted as lines and filled boxes, respectively.