Abstract
Due to high non-maturation rates, arteriovenous fistulas (AVF) frequently require intervention(s) to promote maturation. Endovascular or surgical interventions are often undertaken to salvage non-maturing AVFs. The objective of this study was to compare the impact of surgical versus endovascular interventions to promote AVF maturation on cumulative AVF survival.
We evaluated 89 patients with new AVF placement from a Veterans Affairs population over a 5-year period. Of these, 46 (52%) required intervention(s) to achieve successful maturation for dialysis. 31 patients had surgical revisions and 15 patients had endovascular repairs. We compared cumulative survival between AVFs requiring no intervention, surgical revision, and endovascular intervention to promote AVF maturation.
Cumulative survival was longer in AVFs receiving surgical intervention compared to angioplasty to promote AVF maturation (p=0.05). One year cumulative survival was 86% vs 83% vs 40% for no intervention vs. surgery vs. angioplasty, respectively.
In AVFs that required interventions to promote maturation, AVFs with surgical intervention had longer cumulative survival compared to those AVFs with endovascular intervention. AVFs with surgical intervention to promote maturation had similar one-year cumulative survival to those AVFs that did not require intervention to promote maturation.
Keywords: Arteriovenous Fistula, Vascular Access Intervention, Cumulative Access Survival
Introduction
AVFs that fail to mature, either due to early thrombosis or failure to obtain suitability for dialysis use 1-3, remains the major obstacle to increasing the proportion of dialysis patients with AVFs in the United States. Consequently, we have seen a major effort to aggressively treat and salvage non-developing AVFs to improve AVF maturation outcomes 4-10. Both surgical revision and endovascular repair have been established options for treatment of stenoses in AVFs, with most publications focusing on interventions in previously functioning AVFs and primary patency the main outcome assessed 11-16. However, there are few comparative studies evaluating surgery vs endovascular treatment to salvage non-maturing AVFs. To answer this question, we compared the cumulative survival among AVFs requiring surgical vs endovascular interventions to promote maturation.
Methods
Study Population
A vascular access database from the Cincinnati Veterans Affairs (VA) Medical Center, maintained by a dedicated vascular access coordinator, was queried to identify prevalent hemodialysis patients requiring a new AVF placement from 2005-2010. All patients were under the care of attending VA nephrologists. During this same period, all vascular accesses were created by two dedicated vascular access surgeons with subsequent vascular access surgical revisions or endovascular interventions performed by the same vascular access surgeons or by interventional radiologists.
Vascular Access Management
Pre-operative ultrasound mapping was performed on all patients prior to AVF placement with a minimum threshold of 2.5 mm for the vein and 2.0 mm for the artery used to determine creation of an AVF 17. Patients were evaluated by the surgeon in clinic four to six weeks after creation of an AVF. If there was an abnormality detected on physical exam by the surgeon, the patient had corrective procedures performed either by the surgeon or was referred to interventional radiology. These procedures could include endovascular (angioplasty) or surgical revisions to the AVF. The decision to refer for surgery vs endovascular intervention was based primarily on first availability for operating room time or interventional radiology services. During the study period, interventional radiology services at the Cincinnati VA was available only once a week. In our VA patient population, AVFs are typically allowed to mature for 2 to 3 months before initial cannulation, and permission for initial AVF cannulation is given by the vascular access surgeon or vascular access coordinator, who is an experienced dialysis nurse.
Data collection and analysis
Information related to access history, surgeries, procedures, and outcomes were collected from the vascular access database. The vascular access database included information about vascular access placements and subsequent surgical or endovascular procedures. Demographic information was obtained from the VA Computerized Medical Records System (CPRS).
From the vascular access database, we identified a comprehensive list of AVFs placed in prevalent hemodialysis patients from 2005-2010. We identified 89 patients who had new AVFs placed and were on hemodialysis during this study period. Cumulative access survival was calculated from the time of access creation to permanent failure. The clinical outcome of each AVF was determined from the database.
Demographic and clinical information was collected for each patient including race, presence or absence of diabetes, peripheral vascular disease (PVD), coronary artery disease (CAD), and age. Institutional review board approval from the University of Cincinnati and Cincinnati VA Medical Center Research and Development Committee were obtained prior to initiation of this study.
Statistical Analysis
Data was reported as percentages and means ± S.E., as appropriate. The clinical characteristics were analyzed using contingency table analysis, analysis of variance (ANOVA), and Student’s t-tests. A P-value < 0.05 was considered statistically significant. Cumulative access survival was plotted using Kaplan-Meier survival techniques with patients censored for death, kidney transplant, or end-of follow-up, and the log-rank test used to compare the survival between patient groups. A P-value < 0.05 was considered to be statistically significant. AVFs with primary failures were considered to have survival of 0 days because they were never useable for dialysis. There were 21 primary failures in total: 5 in surgery group, 7 in angioplasty group, and 9 in the group with no intervention. For the analysis comparing cumulative survival between angioplasty and surgical interventions, those patients who had both surgical and angioplasty procedures to promote AVF maturation (5 patients in total) were placed in the angioplasty group for the purposes of the survival analysis. We believe that on a biological level that the vasculature of patients who received both procedures will likely behave more similarly to those with endovascular intervention because of endothelial injury that occurs from balloon angioplasty. All statistical analyses were performed using the JMP® 8.0 (Cary, NC) statistical software package.
Results
Patient Population
Table 1 summarizes the demographic and clinical characteristics of the patient population by type of intervention before maturation or no intervention. The angioplasty group appeared to be a more complex group with a greater proportion of diabetes and peripheral vascular disease, and also a trend towards more coronary artery disease. Age or race did not differ by type of intervention before maturation. Perhaps more importantly approximately 72% of patients in all 3 groups had an AVF at the wrist.
Table 1.
Baseline Demographics by Type of Intervention to Promote Maturation
| No Intervention | Surgery | Angioplasty | P value | |
|---|---|---|---|---|
|
| ||||
| Patients (n=89) | 43 (48%) | 31 (35%) | 15 (17%) | |
|
| ||||
| Age | 67.4±1.8 | 61.9±2.1 | 64.9±3.0 | 0.14 |
|
| ||||
| Race | ||||
| White | 25 (58%) | 21 (68%) | 7 (47%) | 0.38 |
| Black | 18 (42%) | 10 (32%) | 8 (53%) | |
|
| ||||
| Diabetes | ||||
| Yes | 26 (60%) | 16 (52%) | 14 (93%) | 0.009 |
| No | 17 (40%) | 15 (48%) | 1 (7%) | |
|
| ||||
| Coronary Artery Disease | ||||
| Yes | 29 (67%) | 20 (65%) | 14 (93%) | 0.06 |
| No | 14 (33%) | 11 (35%) | 1 (7%) | |
|
| ||||
| Peripheral Vascular Disease | ||||
| Yes | 17 (40%) | 6 (19%) | 10 (67%) | 0.006 |
| No | 26 (60%) | 25 (81%) | 5 (33%) | |
|
| ||||
| Location of Access | ||||
| Upper Arm | 11 (26%) | 10 (32%) | 4 (27%) | 0.81 |
| Forearm | 32 (74%) | 21 (68%) | 11 (73%) | |
Types of Interventions
In total there were 46 total interventions performed to promote AVF maturation (31 surgical and 15 endovascular). Among the 31 surgical interventions, there were 8 correction of accessory veins, 4 conversions to upper arm AVF, 1 interposition graft placement, 2 transpositions, and 16 proximalization of the anastomosis. Among the 15 endovascular interventions, there were 9 angioplasties for juxta-anastomotic lesions and 6 angioplasties for venous stenosis, but non-juxta-anastomotic lesions.
Cumulative Access Survival
Among the 89 patients with new AVF placement, 46 (52%) AVFs required interventions to promote maturation. 31 patients had surgical revisions and 15 patients had endovascular repairs. AVFs with no interventions or surgical interventions had better cumulative survival compared to those AVFs requiring angioplasty (p=0.03) (Figure 1). Cumulative survival was longer in AVFs requiring surgical repair compared to angioplasty to promote AVF maturation (p=0.05). One year cumulative survival was 86% vs 83% vs 40% for no intervention vs. surgery vs. angioplasty groups, respectively.
Figure 1. Cumulative Survival of AVF by Type of Intervention to Promote Maturation.
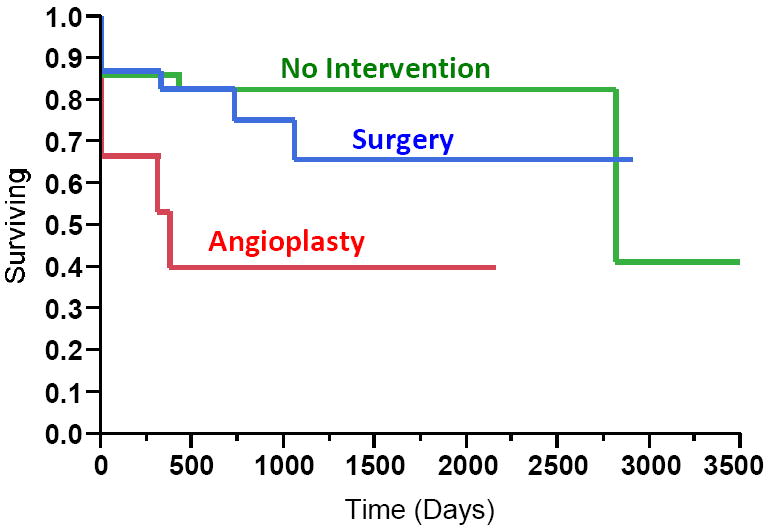
By log-rank test, p=0.03 for all three groups. AVFs with surgical interventions had better cumulative survival compared to angioplasty (p=0.05). One year cumulative survival was 86% vs 83% vs 40% for no intervention vs. surgery vs. angioplasty, respectively.
Discussion
Recent studies have shown high rates of non-maturing AVFs, as high as 60% in a recently published, large, multi-center, randomized clinical trial 1. Non-maturing AVFs frequently have identifiable anatomic abnormalities, most commonly peri-anastomotic stenoses which can identified by physical examination 18-20 and/or angiography 7, 8, 10. Targeted endovascular or surgical interventions to repair these abnormalities are often successful in salvaging non-maturing AVFs making them suitable for dialysis 7-9, 21-23. However, formal comparative studies directly evaluating these two methods in salvaging non-maturing AVFs are lacking.
The few published studies directly comparing surgical vs. endovascular outcomes to treat stenotic or thrombotic AVFs have primarily focused on AVFs that were previously functional for dialysis use 11, 16 and these studies have measured primary patency or restenosis rates. Ito et. al. recently reported that the primary patency rates was significantly lower in endovascular repair of stenotic AVF vs surgical repair 16. Furthermore, Tessitore et al. have reported that the restenosis rate was 2.77 times higher after endovascular vs surgical intervention after pre-emptive repair of juxta-anastomotic stenosis in forearm AVFs 11. There have been only two studies comparing AVF outcomes by the type of intervention to salvage non-maturing AVFs 13, 24. The first study by Long et. al compared outcomes between surgical and endovascular interventions to treat nonmaturing AVFs and reported a one year primary patency of 71% vs. 41% for surgery vs. angioplasty (p<0.02), respectively 13. The second study by Lee et. al reported no difference in cumulative survival in AVFs by type of intervention to promote AVF maturation (p=0.8298) 24. Our current study compares the long-term-outcome between endovascular interventions and surgical repair in non-maturing AVFs requiring intervention to promote maturation, and observed significantly longer cumulative AVF survival in AVFs that had surgical repair compared to endovascular intervention. Moreover, cumulative AVF survival in those AVFs requiring surgical repair to promote AVF maturation was very similar to those AVFs not requiring intervention to promote maturation.
Why might endovascular interventions to promote AVF maturation be associated with shortened cumulative AVF survival compared to surgical repair? The most likely explanation is that these endovascular procedures induce endothelial injury that leads to more aggressive neointimal hyperplasia development, rapid re-stenosis, which eventually leads to AVF failure. In support of this hypothesis, Chang et. al. observed histologically that re-stenotic lesions in AVF after endovascular interventions had greater cellular proliferation activity within the intima and media, as compared with AVFs with primary stenosis 25. Furthermore, in experimental cardiovascular models of vascular injury following angioplasty, a progressive development of inflammation, granulation, extracellular matrix remodeling, smooth muscle cell proliferation and migration occurs, leading to neointimal thickening and restenosis, as well as the inability of the vessels to adequately remodel after injury 26-29. In contrast, surgical repair, specifically creation of a neo-anastomosis, may result in less endothelial perturbation and damage, and consequently have a better long-term survival. Furthermore, our results suggest that surgical repair of nonmaturing AVFs may have similar cumulative survival compared to those AVFs not requiring any intervention for maturation, likely because of less endothelial damage that occurs during the surgical revision.
In the United States endovascular intervention has now replaced surgical therapy as the standard management of vascular access dysfunction, in part due to ease and efficiency of scheduling and minimal invasiveness of the procedure compared to surgery. USRDS data from 2009 shows that angioplasties to treat AVF dysfunction have increased 3-fold from 1998 to 2007 30. Furthermore, recently, endovascular interventions have expanded to include “balloon-assisted maturation” (BAM) for AVF maturation. BAM has been reported in several publications as a routine procedure to promote AVF maturation in non-maturing AVFs 31, 32 and to dilate vessels even prior to AVF creation 33. Until novel and locally-delivered therapies are developed to better treat the endothelial injury that occurs as the result angioplasty, endovascular interventions to treat AVF stenosis will likely continue to be plagued by poor primary patency and cumulative survival compared to surgery11-13. However, at the present time, there are no effective pharmacologic treatments to treat AVF non-maturation, largely due to our limited understanding of the pathophysiology of AVF maturation 5, 34-38, so the standard approach to salvaging nonmaturing AVF will remain the performance of endovascular or surgical interventions. Thus, a major future question is what the ideal approach to treat non-maturing AVFs should be, surgery or endovascular, particularly in distal AVFs with juxta-anastomotic venous lesions, to achieve the best AVF outcomes. Because the data from published observational studies directly comparing treatment (endovascular vs surgery) for stenotic and nonmaturing AVFs with short and long-term AVF outcomes has been inconclusive (but may favor better outcomes with surgical intervention)11-13, this question can only be answered with a well-designed, randomized-controlled trial.
Our present study does have some limitations. First, this was a single center study at a VA medical center, so the results may not be generalizable to all nephrology practices. Second, because our study sample was from VA population, all AVFs created were in males. Therefore, these results may not be generalizable to females, where previous reports have shown females to have lower prevalence of AVFs and smaller vessel sizes 39, 40. Finally, this was a retrospective study, so there may have been undocumented differences in AVFs which received surgical vs. endovascular interventions, other than availability of surgical operating time or interventional services as described in the methods section.
Conclusions
Our results suggest that endovascular interventions to promote AVF maturation may be associated with shorter cumulative AVF survival compared to surgical repair. Furthermore, our results (a) emphasize the importance for further research evaluating the mechanisms of injury following AVF interventions to promote maturation and (b) underscores the urgent need for a randomized-controlled trial that compares surgical vs. endovascular interventions to treat nonmaturing AVFs.
Acknowledgments
Dr. Lee is supported by NIH 5K23DK083528-02 and National Kidney Foundation Franklin McDonald/Fresenius Medical Care Young Investigator Clinical Research Award. Dr. Roy-Chaudhury is supported by NIH 5U01-DK82218, NIH 5U01-DK82218S (ARRA), NIH 5R01-EB004527, NIH 1R21-DK089280-01, a VA Merit Review, two University of Cincinnati NIH/NCCR UL1RR026314 CTSA grants, and industry grants.
Footnotes
Disclosure
Dr. Lee is a consultant for Proteon Therapeutics. Dr. Roy-Chaudhury is on the advisory board/consultant for Pervasis Therapeutics, Inc., Proteon Therapeutics, WL Gore, Bioconnect Systems, Philometron and NanoVasc and receives research support from BioConnect Systems, Shire, Proteon Therapeutics and WL Gore. These funding sources had no involvement in the design or execution of this study.
References
- 1.Dember LM, Beck GJ, Allon M, Delmez JA, Dixon BS, Greenberg A, Himmelfarb J, Vazquez MA, Gassman JJ, Greene T, Radeva MK, Braden GL, Ikizler TA, Rocco MV, Davidson IJ, Kaufman JS, Meyers CM, Kusek JW, Feldman HI for the Dialysis Access Consortium Study G. Effect of Clopidogrel on Early Failure of Arteriovenous Fistulas for Hemodialysis: A Randomized Controlled Trial. JAMA. 2008;299:2164–2171. doi: 10.1001/jama.299.18.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dember LM, Kaufman JS, Beck GJ, Dixon BS, Gassman JJ, Greene T, Himmelfarb J, Hunsicker LG, Kusek JW, Lawson JH, Middleton JP, Radeva M, Schwab SJ, Whiting JF, Feldman HI. Design of the Dialysis Access Consortium (DAC) Clopidogrel Prevention of Early AV Fistula Thrombosis Trial. Clin Trials. 2005;2:413–422. doi: 10.1191/1740774505cn118oa. [DOI] [PubMed] [Google Scholar]
- 3.Roy-Chaudhury P, Sukhatme VP, Cheung AK. Hemodialysis vascular access dysfunction: a cellular and molecular viewpoint. J Am Soc Nephrol. 2006;17:1112–1127. doi: 10.1681/ASN.2005050615. [DOI] [PubMed] [Google Scholar]
- 4.Asif A, Lenz O, Merrill D, Cherla G, Cipleu CD, Ellis R, Francois B, Epstein DL, Pennell P. Percutaneous management of perianastomotic stenosis in arteriovenous fistulae: results of a prospective study. Kidney Int. 2006;69:1904–1909. doi: 10.1038/sj.ki.5000358. [DOI] [PubMed] [Google Scholar]
- 5.Asif A, Roy-Chaudhury P, Beathard GA. Early Arteriovenous Fistula Failure: A Logical Proposal for When and How to Intervene. Clin J Am Soc Nephrol. 2006;1:332–339. doi: 10.2215/CJN.00850805. [DOI] [PubMed] [Google Scholar]
- 6.Beathard GA. Angioplasty for arteriovenous grafts and fistulae. Semin Nephrol. 2002;22:202–210. doi: 10.1053/snep.2002.31739. [DOI] [PubMed] [Google Scholar]
- 7.Beathard GA, Arnold P, Jackson J, Litchfield T. Aggressive treatment of early fistula failure. Kidney Int. 2003;64:1487–1494. doi: 10.1046/j.1523-1755.2003.00210.x. [DOI] [PubMed] [Google Scholar]
- 8.Beathard GA, Settle SM, Shields MW. Salvage of the nonfunctioning arteriovenous fistula. Am J Kidney Dis. 1999;33:910–916. doi: 10.1016/s0272-6386(99)70425-7. [DOI] [PubMed] [Google Scholar]
- 9.Nassar GM, Nguyen B, Rhee E, Achkar K. Endovascular Treatment of the “Failing to Mature” Arteriovenous Fistula. Clin J Am Soc Nephrol. 2006;1:275–280. doi: 10.2215/CJN.00360705. [DOI] [PubMed] [Google Scholar]
- 10.Beathard GA. Fistula salvage by endovascular therapy. Adv Chronic Kidney Dis. 2009;16:339–351. doi: 10.1053/j.ackd.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Tessitore N, Mansueto G, Lipari G, Bedogna V, Tardivo S, Baggio E, Cenzi D, Carbognin G, Poli A, Lupo A. Endovascular versus surgical preemptive repair of forearm arteriovenous fistula juxta-anastomotic stenosis: analysis of data collected prospectively from 1999 to 2004. Clin J Am Soc Nephrol. 2006;1:448–454. doi: 10.2215/CJN.01351005. [DOI] [PubMed] [Google Scholar]
- 12.Lipari G, Tessitore N, Poli A, Bedogna V, Impedovo A, Lupo A, Baggio E. Outcomes of surgical revision of stenosed and thrombosed forearm arteriovenous fistulae for haemodialysis. Nephrol Dial Transplant. 2007;22:2605–2612. doi: 10.1093/ndt/gfm239. [DOI] [PubMed] [Google Scholar]
- 13.Long B, Brichart N, Lermusiaux P, Turmel-Rodrigues L, Artru B, Boutin JM, Pengloan J, Bertrand P, Bruyere F. Management of perianastomotic stenosis of direct wrist autogenous radial-cephalic arteriovenous accesses for dialysis. Journal of vascular surgery : official publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, North American Chapter. 2011;53:108–114. doi: 10.1016/j.jvs.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Cohen A, Korzets A, Neyman H, Ori Y, Baytner S, Belenky A, Knieznik M, Bachar GN, Atar E. Endovascular interventions of juxtaanastomotic stenoses and thromboses of hemodialysis arteriovenous fistulas. Journal of vascular and interventional radiology : JVIR. 2009;20:66–70. doi: 10.1016/j.jvir.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Tordoir JH, Bode AS, Peppelenbosch N, van der Sande FM, de Haan MW. Surgical or endovascular repair of thrombosed dialysis vascular access: is there any evidence? Journal of vascular surgery : official publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, North American Chapter. 2009;50:953–956. doi: 10.1016/j.jvs.2009.06.058. [DOI] [PubMed] [Google Scholar]
- 16.Ito Y, Sato T, Okada R, Nakamura N, Kimura K, Takahashi R, Miwa N, Sakurai H, Tsuboi M, Kasuga H. Comparison of clinical effectiveness between surgical and endovascular treatment for thrombotic obstruction in hemodialysis access. The journal of vascular access. 2011;12:63–66. doi: 10.5301/jva.2010.5983. [DOI] [PubMed] [Google Scholar]
- 17.Clinical Practice Guidelines for Vascular Access. Am J Kidney Dis. 2006;48:S176–S273. doi: 10.1053/j.ajkd.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 18.Asif A, Leon C, Orozco-Vargas LC, Krishnamurthy G, Choi KL, Mercado C, Merrill D, Thomas I, Salman L, Artikov S, Bourgoignie JJ. Accuracy of physical examination in the detection of arteriovenous fistula stenosis. Clin J Am Soc Nephrol. 2007;2:1191–1194. doi: 10.2215/CJN.02400607. [DOI] [PubMed] [Google Scholar]
- 19.Leon C, Asif A. Physical examination of arteriovenous fistulae by a renal fellow: does it compare favorably to an experienced interventionalist? Seminars in dialysis. 2008;21:557–560. doi: 10.1111/j.1525-139X.2008.00477.x. [DOI] [PubMed] [Google Scholar]
- 20.Beathard GA. An algorithm for the physical examination of early fistula failure. Semin Dial. 2005;18:331–335. doi: 10.1111/j.1525-139X.2005.18314.x. [DOI] [PubMed] [Google Scholar]
- 21.Shin SW, Do YS, Choo SW, Lieu WC, Choo IW. Salvage of immature arteriovenous fistulas with percutaneous transluminal angioplasty. Cardiovasc Intervent Radiol. 2005;28:434–438. doi: 10.1007/s00270-003-0211-x. [DOI] [PubMed] [Google Scholar]
- 22.Clark TW, Cohen RA, Kwak A, Markmann JF, Stavropoulos SW, Patel AA, Soulen MC, Mondschein JI, Kobrin S, Shlansky-Goldberg RD, Trerotola SO. Salvage of nonmaturing native fistulas by using angioplasty. Radiology. 2007;242:286–292. doi: 10.1148/radiol.2421051718. [DOI] [PubMed] [Google Scholar]
- 23.Turmel-Rodrigues L, Mouton A, Birmele B, Billaux L, Ammar N, Grezard O, Hauss S, Pengloan J. Salvage of immature forearm fistulas for haemodialysis by interventional radiology. Nephrol Dial Transplant. 2001;16:2365–2371. doi: 10.1093/ndt/16.12.2365. [DOI] [PubMed] [Google Scholar]
- 24.Lee T, Ullah A, Allon M, Succop P, El-Khatib M, Munda R, Roy-Chaudhury P. Decreased cumulative access survival in arteriovenous fistulas requiring interventions to promote maturation. Clinical journal of the American Society of Nephrology : CJASN. 2011;6:575–581. doi: 10.2215/CJN.06630810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang CJ, Ko PJ, Hsu LA, Ko YS, Ko YL, Chen CF, Huang CC, Hsu TS, Lee YS, Pang JH. Highly increased cell proliferation activity in the restenotic hemodialysis vascular access after percutaneous transluminal angioplasty: implication in prevention of restenosis. Am J Kidney Dis. 2004;43:74–84. doi: 10.1053/j.ajkd.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 26.Inoue T, Node K. Molecular basis of restenosis and novel issues of drug-eluting stents. Circ J. 2009;73:615–621. doi: 10.1253/circj.cj-09-0059. [DOI] [PubMed] [Google Scholar]
- 27.Okamoto E, Couse T, De Leon H, Vinten-Johansen J, Goodman RB, Scott NA, Wilcox JN. Perivascular inflammation after balloon angioplasty of porcine coronary arteries. Circulation. 2001;104:2228–2235. doi: 10.1161/hc4301.097195. [DOI] [PubMed] [Google Scholar]
- 28.Nakatani M, Takeyama Y, Shibata M, Yorozuya M, Suzuki H, Koba S, Katagiri T. Mechanisms of restenosis after coronary intervention: difference between plain old balloon angioplasty and stenting. Cardiovasc Pathol. 2003;12:40–48. doi: 10.1016/s1054-8807(02)00135-7. [DOI] [PubMed] [Google Scholar]
- 29.Libby P, Tanaka H. The molecular bases of restenosis. Prog Cardiovasc Dis. 1997;40:97–106. doi: 10.1016/s0033-0620(97)80002-3. [DOI] [PubMed] [Google Scholar]
- 30.U.S. Renal Data System, USRDS 2009. Annual Data Report: Atlas of CKD and ESRD in the United States, National Institutes of Health. National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2009. [Google Scholar]
- 31.Miller GA, Goel N, Khariton A, Friedman A, Savransky Y, Trusov I, Jotwani K, Savransky E, Preddie D, Arnold WP. Aggressive approach to salvage non-maturing arteriovenous fistulae: a retrospective study with follow-up. J Vasc Access. 2009;10:183–191. doi: 10.1177/112972980901000309. [DOI] [PubMed] [Google Scholar]
- 32.Samett EJ, Hastie J, Chopra PR, Pradhan S, Ahmad I, Chiramel T, Joseph R. Augmented balloon-assisted maturation (aBAM) for nonmaturing dialysis arteriovenous fistula. The journal of vascular access. 2011;12:9–12. doi: 10.5301/jva.2010.6018. [DOI] [PubMed] [Google Scholar]
- 33.De Marco Garcia LP, Davila-Santini LR, Feng Q, Calderin J, Krishnasastry KV, Panetta TF. Primary balloon angioplasty plus balloon angioplasty maturation to upgrade small-caliber veins (<3 mm) for arteriovenous fistulas. Journal of vascular surgery : official publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, North American Chapter. 2010;52:139–144. doi: 10.1016/j.jvs.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Roy-Chaudhury P, Lee TC. Vascular stenosis: biology and interventions. Curr Opin Nephrol Hypertens. 2007;16:516–522. doi: 10.1097/MNH.0b013e3282efa57f. [DOI] [PubMed] [Google Scholar]
- 35.Roy-Chaudhury P, Spergel LM, Besarab A, Asif A, Ravani P. Biology of arteriovenous fistula failure. J Nephrol. 2007;20:150–163. [PubMed] [Google Scholar]
- 36.Lee T, Roy-Chaudhury P. Advances and new frontiers in the pathophysiology of venous neointimal hyperplasia and dialysis access stenosis. Adv Chronic Kidney Dis. 2009;16:329–338. doi: 10.1053/j.ackd.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diskin CJ. Novel insights into the pathobiology of the vascular access - do they translate into improved care? Blood Purif. 2010;29:216–229. doi: 10.1159/000245650. [DOI] [PubMed] [Google Scholar]
- 38.Dixon BS. Why don’t fistulas mature? Kidney Int. 2006;70:1413–1422. doi: 10.1038/sj.ki.5001747. [DOI] [PubMed] [Google Scholar]
- 39.Miller CD, Robbin ML, Allon M. Gender differences in outcomes of arteriovenous fistulas in hemodialysis patients. Kidney Int. 2003;63:346–352. doi: 10.1046/j.1523-1755.2003.00740.x. [DOI] [PubMed] [Google Scholar]
- 40.Miller PE, Tolwani A, Luscy CP, Deierhoi MH, Bailey R, Redden DT, Allon M. Predictors of adequacy of arteriovenous fistulas in hemodialysis patients. Kidney Int. 1999;56:275–280. doi: 10.1046/j.1523-1755.1999.00515.x. [DOI] [PubMed] [Google Scholar]


