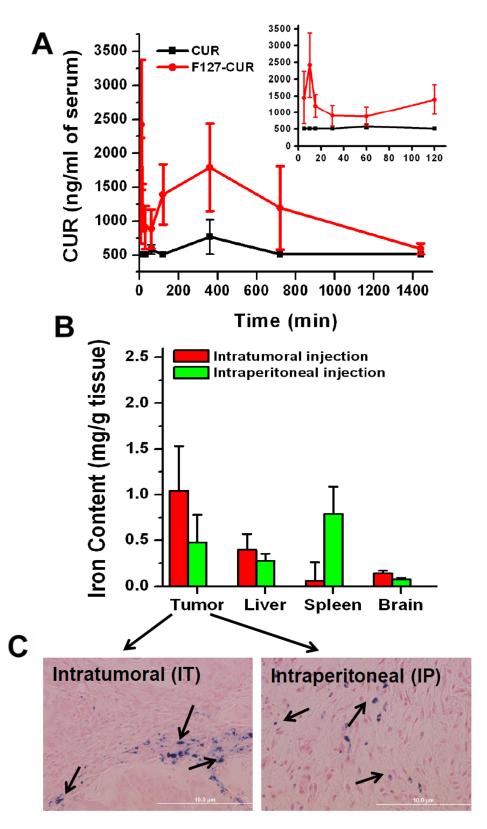
Figure 5.
Serum bioavailability and biodistribution of MNP-CUR formulation. (A) Comparative serum bioavailability of curcumin (CUR) and MNP-CUR formulations in a tumor bearing mice model. Enlarged 5, 10, 15, 30, 60 and 120 min bioavailability data is shown as inset. Curcumin (CUR) and MNP-CUR (50 μg per mice) were administered via intraperitoneal injection and blood samples from tail bleed were collected at different time points. CUR levels in serum was determined using a Dionex HPLC instrument equipped with an Acclaim Polar Advantage II C18 column (3 μm 120 Å 4.6 × 150 mm) with the RS variable wavelength detector at 420 nm. (B) Comparative biodistribution in tumor, liver, spleen and brain tissues after intratumoral and intraperitoneal administration of MNP-CUR formulation. Mice were treated with MNP-CUR particles (20 μg per mice) and iron content of particles in tissue was estimated using 1,10-phenanthroline colorimetric method. (C) Visual evidence of nanoparticles in tumor tissue was detected with Prussian blue staining method. Images were captured using an Olympus 1X71 microscope equipped with a DP71 camera (Olympus).