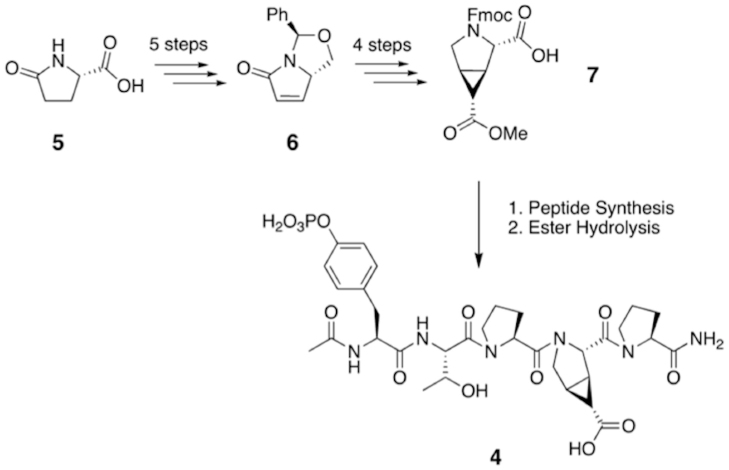
Figure 3. Synthetic route to generate 4.
L-pyroglutamic acid, 5, was used to generate the unsaturated bicyclic ring system 6 over a series of 5 steps. 6 then yielded a fully protected cyp-ptE, 7, suitable for peptide synthesis. 7 was incorporated into the pentapeptide sequence pYTPXP, where X denotes the variable amino acid position, using standard solid phase peptide synthesis methodology. Treatment of the purified peptide with NaOH converted the methyl ester to the desired acid, yielding 4.