Abstract
Purpose
The present study hypothesized that simple, everyday physical activity (EPA) would decline with advancing age; that women would have a more favorable EPA profile than would men; and that EPA would have a survival benefit.
Design and Methods
Community-dwelling participants (aged 80–98 years, n = 198) wore mechanical actigraphs in order for EPA to be assessed. Individuals were classified as active, inactive, and sedentary based on their level of EPA exhibited over a substantial part of the day. Survival status was available at approximately 2 years.
Results
Mean EPA scores decreased with advancing age and, in contrast to men, women in their early eighties appeared to be protected from declining EPA. This partially supported the hypothesis that women would have a more favorable EPA profile. What is most important is that mean EPA scores predicted mortality. Moreover, when compared with their less sedentary counterparts, sedentary adults were more than three times as likely to be deceased 2 years later.
Implications
Researchers need to conduct new trials to determine whether or how physical activity is associated with mortality.
Keywords: Death, Gender, Longevity, Sedentary, Survival
Although a young person may be unable to conceive of a sedentary life, some older adults give up any activity that is not essential. For them, a typical day can involve sitting in stillness for long hours, even avoiding unnecessary steps to another room. At a very basic level, many negative consequences can follow from a lack of physical movement, as is well documented in the literature on physical restraint: skin breaks down; extremities swell (edema); muscles weaken and shorten (contractures); and abnormal changes occur in metabolic rates, body chemistry, and blood volume (e.g., Mott, Poole, & Kenrick, 2005).
A lack of physical activity is implicated in many conditions and problems, and it has even been shown to predict mortality (e.g., Lee, Sesso, Oguma, & Paffenbarger, 2004). For example, among 302 older, high-functioning, community-dwelling adults, researchers found that low physical activity as measured by energy expenditure or metabolic rate was associated with mortality 6 years later, after they adjusted for such confounding factors as health and education (Manini et al., 2006). This association between low activity and mortality has primarily been demonstrated in studies that measure self-reported activity (Andersen, Schnohr, Schroll, & Hein, 2000; Kaplan, Seeman, Cohen, Knudsen, & Guralnik, 1987; Stessman, Maaravi, Hammerman-Rozenberg, & Cohen, 2000).
Dishman, Washburn, and Schoeller (2001) reviewed the plethora of self-report activity measures, the ways in which they have been used, and their limitations. Notable drawbacks of such self-report measures include unreliability and biased overestimates (Hoyt & Kerns, 1999; Sallis & Saelens, 2000). Less subject to bias are objective mechanical motion recorders, such as pedometers, actometers, and actigraphs, which capture the expansiveness, frequency, duration, and amplitude of simple, physical movement. This allows for a quantification of physical movement as it occurs (or does not occur) within one’s daily endeavors. Although mechanical recorders have primarily been used with infants and children (Chipperfield & Eaton, 1992; Eaton, Chipperfield, & Singbeil, 1989), they are beginning to be applied to the study of older adults (e.g., Fukukawa et al., 2004). Because in later life some individuals are extremely sedentary, these instruments may be especially valuable in that they reliably capture inactivity (Esliger, Copeland, Barnes, & Tremblay, 2005).
Age-Related Declines and Gender Differences in Physical Activity
Physical activity clearly declines with age, the decrease having been documented from infancy to young childhood (Eaton, 1994) and more so from 11 to 18 years of age (Brodersen, Steptoe, Boniface, & Wardle, 2007; Schoenborn, 1986; Trost, Pate, et al., 2002). A relationship between age and activity is also clearly illustrated over the life course (Trost, Owen, Bauman, Sallis, & Brown, 2002), with age being inversely related to physical activity over 5 and 8 years (Armstrong & Morgan, 1998; Sihvonen, Rantanen, & Heikkinen, 1998).
Just as the literature provides consensus regarding age-related declines in physical activity, gender differences are also found. Beginning early in life, boys are more active than girls (Eaton & Enns, 1986), and this gender gap is evident during childhood and adolescence (Trost, Pate, et al., 2002). Even among older populations, women are less physically active than men (e.g., Curtis, White, & McPherson, 2000; Trost, Owen, et al., 2002), and they are less likely to meet the frequency and duration standards of physical activity (Zizzi et al., 2006). Moreover, in the Nottingham Longitudinal Study (n = 1,042, aged 65 years or older at baseline), Bennet (1998) found that men maintained higher levels of outdoor activity than women did over an 8-year time period. However, the gender difference in Bennet’s study was reversed for indoor activity: Women had higher activity than men. Likewise, in another study, although men reported higher energy expenditure than women did for yard work, women reported higher energy expenditure for household work (Kolbe-Alexander, Lambert, Biletnikoff-Harkins, & Ekelund, 2006). Perhaps when activity is assessed indoors, women are more active than men, suggesting that, in late life, there is likely to be a reversal of the well-established gender difference. It is also possible that gender plays a moderating role (Curtis et al.) such that men and women differ in the gradient of age-related declines in physical activity.
The Present Study: Aging and Everyday Physical Activity
The present study of a representative sample of older, community-dwelling adults conceived of simple everyday physical activity (EPA) in a way that captures the small everyday physical movements involved in getting around the house, distinguishing this activity from more periodic, intense forms of activity. Thus EPA shares conceptual similarity with the construct of nonexercise activity thermogenesis, which involves energy expended through activities such as standing or typing (Levine et al., 2005). In the present study I assessed EPA by having participants wear actigraphs as they went about their normal daily activities, providing a rich source of data to quantify physical movement and to identify sedentary individuals.
More generally, the data allowed for the development of a precise, descriptive profile of EPA in late life. This included a consideration of whether simple EPA declines with age. In keeping with the broad literature on activity and exercise (Brach, Simonsick, Kritchevsky, Yaffe, & Newman, 2004), I hypothesized that very old adults (85 years of age or older) would have a lower EPA level than would their younger counterparts (younger than 85 years). To develop the descriptive profile, I also assessed gender differences, considering whether men and women differ in (a) overall mean levels of EPA, (b) the likelihood of being categorized above or below certain predefined activity thresholds as described later in more detail, and (c) the age gradient for EPA, that is, the strength of the negative relationship. In contrast to the gender difference that has typically emerged (men being more active than women), I expected a reversal, given the largely indoor context in which EPA was assessed. That is, I expected a more positive EPA profile for women than for men.
In addition to developing a descriptive profile, a second objective of the study was to consider the possible consequences of EPA for survival. Studies that have established a link between activity or exercise and mortality have focused only on men (Bijnen et al., 1998; Hakim et al., 1998; Lee, Sesso, Oguma, & Pattenbarger, 2004) or women (Kushi et al., 1997) and have been restricted to specialized samples such as high-functioning individuals (Lee et al.; Manini et al., 2006) or participants in a heart program (Hakim et al.). Moreover, although a few researchers have examined older adults in their 80s or even into their 90s (e.g., Bijnen et al.; Hakim et al.; Kaplan et al., 1987), studies that have assessed the relationship between physical activity and mortality in a representative sample of very old adults are rare, if they exist at all. The existing studies have also typically measured self-reported activity (e.g., Kaplan et al.), not EPA as defined and precisely assessed in this study.
Methods
Sample Selection: The Study of Everyday Physical Activity
As described in detail elsewhere, the Study of Everyday Physical Activity is a satellite study of the larger Aging in Manitoba (AIM) Longitudinal Project, which contains data on nearly 9,000 older adults who were interviewed over a 35-year period (Chipperfield et al., 2006; Chipperfield & Perry, 2006). The original wave of AIM participants was interviewed in 1971, and new waves were added in 1976 and 1983. Rigorous stratified randomization techniques were used to select participants for each new wave, resulting in probability samples stratified by age, gender, and region. Follow-up interviews were also conducted with all surviving individuals at six subsequent points in time. Previous analyses show that the sampling procedures successfully achieved representativeness and minimized selection bias and selective attrition (Chipperfield, Havens, & Doig, 1997).
In 2003, the EPA sample was drawn from the pool of AIM respondents who participated in the 2001 follow-up interview. Participants were eligible for the EPA if they (a) lived in or very near the major cities in Manitoba, Canada; (b) needed no or only little assistance to complete interviews; and (c) had satisfactory comprehension of English. Interviews were conducted with 232 participants, with actigraph data being obtained from 198 individuals. Excluded from the EPA sample were 34 individuals because their data were unusable (n = 7), they refused to wear the actigraph (n = 12), or they participated at a time when an actigraph was unavailable (n = 15). These 34 nonresponders did not differ from responders on survival status or on other major independent variables as subsequently described (age, income, education, gender, severity of health conditions, positive affect), with the exception that nonrespondents had significantly greater health-related restriction, 11.2 versus 7.8, t(225) = 2.49, p = .01.
Study Procedure
The study procedures for the EPA involved two visits to participants’ homes, typically over a 2-day time period. On Visit 1, prior to conducting an in-depth interview, an interviewer placed an actigraph on the participant’s nondominant wrist, much like a wristwatch, and recorded the start time. Placement of the actigraph on the wrist (rather than the ankle or waist) is consistent with past approaches (Steele et al., 2003) and captures the upper-body movements involved in such everyday activities that occur while sitting (e.g., sewing, playing cards) or standing (e.g., dishwashing, moving about the house). Wrist placement also made it simpler for participants to remove and reattach the actigraphs if this became necessary during the study. Interviewers encouraged participants to wear the actigraph continuously, although instructions were provided on how to remove and reattach it and where to record the times and reasons this was done. Interviewers also encouraged participants to go about their daily activities as they normally would.
On the next day for Visit 2, interviewers returned to remove the actigraph, record the end time, and ask several questions to determine, for example, the times at which participants went to bed at night and got up in the morning. If a Visit 2 appointment could not be scheduled for the next day, the participant was asked to remove the actigraph (approximately 24 hours after the start time) and to note the exact time. The actigraph data were downloaded after each use and the actigraph was reinitialized for use by the next participant.
Measures
Sample Characteristics
The interviewer obtained sample characteristics for the 198 participants during the interview. Participants were between 80 and 98 years of age (M = 85.0 yrs, SD = 4.39); the majority were women (63.1%). On average, participants reported having 10.5 years of education and an estimated total personal annual income of $21,369.00 (dollars in Canadian currency).
Physical Status
Physical status was determined by creating a Severity of Chronic Conditions (SCC) score for each participant. This involved first establishing the presence (yes, no) of 21 chronic conditions (M = 5.2) and then deriving a score to reflect the severity of them (Chipperfield et al., 2006). Where possible,1 this was done by borrowing severity rankings provided by medical students or residents for illnesses on the Seriousness of Illness Rating Scale (Rosenberg, Hayes, & Peterson, 1987). For the present study, a total SCC score was created for each participant by summing over the rankings that corresponded to each of his or her reported chronic conditions (M = 54.13, SD = 28.19). For example, if a person had arthritis (ranked 13th), Alzheimer’s disease (ranked 17th), and diabetes (ranked 18th), the SCC score would reflect the sum of the severities associated with these three conditions (i.e., 48.0). This SCC measure of morbidity correlated with a single self-rated health item also available in the data set; r(198) = −.31, p < .01.
Functional Status
Functional status was assessed by having the interviewer ask participants how often during the past year each of their existing chronic health problems restricted the things they were able to do (1 = never, 4 = almost always). By summing over the restrictiveness of each reported health condition, I obtained a health-related restriction (HHR) score in which higher scores indicated poorer functional status. This HHR measure was strongly negatively correlated to a measure of independence on basic activities of daily living, r(198) = −.42, p < .01, that was obtained 2 years earlier.
Psychological status
Psychological status was determined by examining positive affect, which involved obtaining the mean frequency (0 = never, 3 = sometimes, 6 = almost always) with which respondents reported feeling six emotions (happiness, gratitude, hope, contentment, pride, and relief) over the past 2 days (M = 2.78, SD = 1.10). As reported elsewhere (Chipperfield, Perry, & Weiner, 2003), this composite positive affect measure has adequate internal consistency (α = .61), and it correlated (r = .38, p < .01) with the 20-item Life Satisfaction Index A measure of Neugarten, Havighurst, and Tobin (1961; α = .74).
Everyday Physical Activity
EPA was assessed using Actigraph uniaxial accelerometers (Model 7164, developed by MTI Health Services, 2000). Powered by a 2,430 coin cell lithium battery, these actigraphs assess the intensity of movement in the vertical plane, the vertical accelerations ranging in magnitude from 0.05 to 2.0 G, with bandwidths limited to a frequency response from 0.25 to 2.50 Hz. Specifying these parameters is done in an attempt to detect human body movement and reject motion from other sources.
Measuring only 2.0 × 1.6 × 0.6 in. (5.08 cm × 4.06 cm × 1.52 cm) and weighing 1.5 oz (42.52 g), the actigraph can be worn comfortably while it obtains a continuous recording of activity from acceleration signal magnitudes that are summed over a user-defined cycle interval, which was specified in the present study as 1 minute. At the end of every 1-minute cycle, the 10 acceleration signals captured per second are summed, thereby combining 600 accelerations (10 × 60 seconds) before the integrator is reset to zero. These 1-minute acceleration scores, which can be interpreted as the amount of activity per minute, are stored in memory and are easily downloaded onto a computer.
For the present purposes, I generated a continuous EPA score for each participant after excluding acceleration data that were associated with the time a person spent in bed at night or not wearing the actigraph (see Chipperfield et al., 2006). In particular, I divided the sum of a participant’s 1-minute acceleration scores by the total number of acceleration scores obtained for that participant (M = 763.88, SD = 320.74; range = 78–1,745).
In addition to this continuous EPA score, I used a three-step process to create dichotomous, categorical variables that distinguished between active and inactive participants. First, as suggested by Steele and colleagues (2003), acceleration scores were classified on the basis of whether they met or exceeded predetermined thresholds, defined in this study as standard deviation units above and below the grand EPA mean. By determining whether every 1-minute acceleration score for each participant exceeded a given threshold, I coded each score as a “burst of activity” if it exceeded ≥ 1 SD (1,085) or ≥ 2 SDs (1,405) or as an “episode of quiescence” if it exceeded ≤ 1 SD (443) or ≤ 2 SDs (122). Second, I determined the proportion of the day participants exhibited bursts of activity or episodes of quiescence by dividing the total number of activity bursts or quiescent episodes by the total number of acceleration scores obtained for a given individual. Third, I classified participants as moderately active and extremely active if, for a substantial proportion of their day (defined as ≥ 30%), they exhibited bursts of activity that met or exceeded 1 SD and 2 SDs above the mean, respectively. In a similar way, I classified participants as moderately inactive and extremely inactive if they exhibited episodes of quiescence that met or exceeded 1 SD and 2 SDs below the mean, respectively, for at least 30% of their day.
Finally, participants were also classified as sedentary on the basis of a complete absence of any physical movement (i.e., acceleration scores of zero) for a substantial proportion of the day (≥30 %). Although there was some overlap between the classification of individuals as extremely inactive or sedentary, the sedentary classification provides a more precise indicator of the absolute lack of EPA. It also offers an important reference point for comparison with other studies in individuals are classified as sedentary.
Table 1, which is described in more detail later, shows that nearly 20% of the participants in the total sample were classified as sedentary. Fewer people in the overall sample were classified as being active (having exhibited bursts of activity) relative to inactive (having exhibited episodes of quiescence). Whereas only 20% of participants were extremely active, nearly 70% were extremely inactive.
Table 1.
Percentages of Participants Categorized as Active, Inactive, and Sedentary
| Total Sample | Age in Years
|
Gender
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| <85 | ≥85 | χ2 | p | Male | Female | χ2 | p | ||
| Category | |||||||||
| Moderately Active | 35 | 42 | 27 | 4.5 | .02* | 32 | 37 | 0.57 | .23 |
| Extremely Active | 20 | 23 | 16 | 1.8 | .09† | 16 | 22 | 0.78 | .19 |
| Moderately Inactive | 97 | 95 | 99 | 2.4 | .06† | 97 | 97 | 0.03 | .43 |
| Extremely Inactive | 69 | 61 | 78 | 6.5 | .01* | 77 | 65 | 3.07 | .04* |
| Sedentary | 19 | 15 | 23 | 2.4 | .06† | 23 | 16 | 1.61 | .10† |
Notes: EPA = everyday physical activity; numbers for the table are as follows. Total sample, n = 198; <85 years, n = 103; ≥85 years, n = 95; male gender, n =75; female, n = 125. Moderately and extremely active participants exhibited bursts of activity that met or exceeded 1 SD and 2 SD above the mean, respectively. Moderately and extremely inactive participants exhibited episodes of quiescence that met or exceeded 1 SD and 2 SD below the mean, respectively. Sedentary participants exhibited a complete absence of physical movement.
p ≤ .05.
p ≤ .10.
Reliability
Although the reliability and validity of actigraphs are well established (e.g., Melanson & Freedson, 1995; Welk, Schaben, & Morrow, 2004), I conducted several analyses to provide further supportive evidence. First, participants’ self-reports of activity on the study day showed that very few participants (10.7%) stated they had atypical activity that was either lower or higher than usual. Moreover, those who did report atypical activity levels on the study day did not differ on their EPA score from those who reported their activity was typical on that day, t(194) = 1.22, p = −.22. Second, because the reliability of a 1-day EPA measure would be threatened by a day-of-week effect that has been reported in prior work with younger adults (Lee et al., 2004), I conducted a 2 × 2 analysis of variance to examine the effect of day of week (weekend vs weekday) by gender (male or female) on EPA. The effects were nonsignificant for day, F(1, 197) = 0.28, p = .60; gender, F(1, 197) = −0 .08, p = .93; and Day × Gender, F(1, 197) = 0.33, p = .57. This suggests that EPA is similar across weekdays and weekend days. Third, a smaller study that was conducted approximately 1 year later showed stability over days when participants wore actigraphs for 1, 2, or 3 days (Lambert, 2006). Finally, because a subset of respondents participated in both the present study and this smaller study (n = 68), test–retest reliability could be examined. Time 1 and Time 2 EPA scores were highly correlated, r = .77, p < .01, and they did not differ significantly, t(67) = −1.69, p = .10.
Mortality
Information was obtained to identify each participant’s survival status (deceased or alive) in the subsequent 2 years after the EPA assessment. Those participants who had died were located by searching hospital records and the Manitoba Vital Statistics database. Approval was obtained from the Heath Information and Privacy Committee to link the interview data with these vital statistics. Because the status of 7 individuals could not be tracked, the subsequent mortality analyses were reduced to 191 individuals (15 = deceased, 176 = alive).
Results
A descriptive profile of EPA in later life was developed by examining several indicators of EPA, including the mean EPA score and the categorical variables of active, inactive, and sedentary. I used a value of p = .05 and two-tailed tests except when I applied one-tailed tests to assess the unidirectional hypotheses regarding age, gender, and the relationship between EPA and survival.
A Descriptive Profile: Gender and Age Differences in EPA
Mean EPA Scores
Less favorable EPA profiles were found for old-old adults relative to young-old adults (<85 years of age) in that they had a significantly lower mean EPA score, t(196) = 3.03, p < .01. Although women had a relatively higher mean EPA score than men (M = 769 vs M = 756), the difference was not significant; t(196) = −0.27, p = .79.
Categorical Threshold Variables
Consistent with the findings from the analysis of mean EPA scores, the categorical threshold data shown in Table 1 revealed less favorable activity profiles for the old-old (i.e., those <85 years of age) relative to the young-old adults (i.e., those 85+ year of age). A smaller proportion of the old-old relative to the young-old adults were active, and the age difference was significant for moderately active participants (27% vs 42%). Likewise, relative to the young-old adults, old-old adults were more likely to be extremely inactive (78% vs 61%), and they were marginally more likely to be sedentary (15% vs 23%).
No gender differences emerged for the proportions of participants who were classified as either moderately or extremely active. However, men were more likely than women to be classified as extremely inactive (77% vs 65%) Men were also (marginally) more likely to be classified as being sedentary (23% vs 16%), providing additional support for a reversal of the past reported gender difference that has shown men to be more active than women.
The Relationship Between EPA and Advancing Age
Consistent with the prior analyses that showed older adults had less positive EPA profiles than younger adults, this same pattern emerged in an examination of the correlations between age and EPA that was done separately for both young-old individuals (<85 years) and old-old individuals (≥85 years). The predicted negative relationship between age and EPA was of equal magnitude for old-old men and women (r = −.31 vs r = −.27), but it differed for young-old men and women (r = −.31 vs r = −.11), suggesting a gender difference in the age gradient of EPA. To consider the potential moderating effect of gender in patterns of EPA among the young-old adults, I regressed EPA on age, gender, and the (centred) Age × Gender interaction.
Significant effects emerged for age (t = −2.25, p = .01), gender (t = −2.14, p = .01), and the Age × Gender interaction (t = 2.14, p = .04), confirming that gender acts as a moderator in the relationship between age and EPA. Figure 1 shows the superimposed slopes obtained from regression analyses testing the effects of age separately for men and women (and separately for young-old and old-old individuals). Among the young-old individuals, as expected, the pattern of lower EPA scores with advancing age was found for men (B = −76.7 β = −0.31, t = 1.92, p = .03) but not for women (B = 17.83, β = 0.08, t = 0.63, p = .53). Figure 1 also illustrates the parallel slopes for the old-old men and women.
Figure 1.
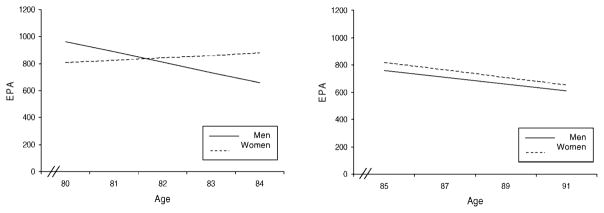
Age-related patterns of everyday physical activity (EPA) for men and women.
The Relationship Between EPA and Mortality
A logistic regression was conducted to examine whether EPA predicted the probability of dying within a 2-year follow-up period. Although a proportional hazards approach that estimates incidence rates would have advantages (e.g., greater power), logistic regression is suited to establishing the odds of dying, given variations in levels of activity. Prior to examining the odds of dying, preliminary analyses were conducted to identify potential covariates. A conservative approach was adopted to include as a predictor any variables that correlated at or above .10 with either EPA or mortality. From among age, education, income, functional status (health-related restriction), physical status (severity of chronic conditions), and psychological status (positive affect), I excluded education and income as covariates because they were uncorrelated with mean EPA or mortality.
The main logistic regression analyses of the upper threshold data found no support to show a higher probability of dying among those moderately active or extremely active individuals relative to their less active counterparts (β = −0.679, p = .32; β = −1.20, p=.26). In contrast, however, the mean (continuous) EPA score significantly predicted mortality 2 years later. Decreasing EPA levels were associated with an increased probability of dying (β = −.002, Wald = 3.34, p = .03), even after controlling for age (β = 0.007, p = .91), health status (β = 0.004, p = .76), functional status (β = 0.036, p = .47), and psychological status (β = −0.244, p = .36). As illustrated in Figure 2, support was also found in a separate logistic regression to show that those classified as sedentary were significantly more likely to die in the subsequent 2 years than were their less sedentary counterparts (19% vs 5%, β = 1.42, Wald = 4.36, p = .019, odds ratio = 3.44). Interestingly, separate analyses of men and women suggested differences in their patterns: only 10.5% (2 of 19) of sedentary women were deceased 2 years later, whereas 29% (5 of 17) of sedentary men were deceased (Figure 2). The numbers are too small to conduct unbiased statistical tests of this difference, but it may be notable that the magnitude of the EPA–mortality association is relatively stronger for men (r = −.21) than it is for women (r = −.11).
Figure 2.
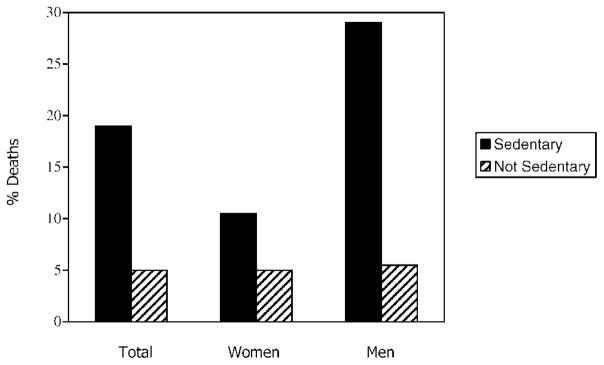
Percentages of sedentary versus active men and women who were deceased 2 years later.
Supplemental Analyses
Although the main regression models controlled for physical, functional, and psychological status, it is possible that these measures did not fully capture the poorer status of those individuals who were sedentary (or who had low overall mean EPA scores). Thus, I tested alternate models, replacing the physical, functional, and psychological status variables with alternate measures of self-rated health, basic activities of daily living, and life satisfaction. Even with these alternate controls (as well as age), the odds ratio of dying was significantly greater with lower levels of overall mean EPA (β = −0.002, Wald = 3.31, p = .04). A significant odds ratio was also found when comparing sedentary versus less sedentary adults (β = 1.38, Wald = 5.16, p = .01).
Discussion
This study provided a precise, quantifiable account of the extent to which older adults are physically active or inactive, resulting in three major conclusions. First, as I predicted, the data unequivocally demonstrate a negative relationship between EPA and advancing age. This is congruent with longitudinal data documenting age-related declines in self-reported physical activity (Slingerland et al., 2007). Given the advanced age of participants, it is not surprising that the mean EPA was low. Although the people in this study were generally moving around, albeit slowly, inactivity was also common: 15% of the young-old adults were classified as sedentary, rising to 23% of old-old participants. Nevertheless, these data provide a more positive profile than prior research based on self-reported activity, which shows as many as 35% of adults are classified as sedentary (Schroll, 2003; Mattiasson-Nilo et al., 1990).
Second, the data showed a more favorable EPA profile for women, providing partial support for the gender hypothesis. Whereas the EPA decline occurred for men throughout their 80s and 90s, it did not begin for women until the age of 85. Perhaps the demands of day-to-day caregiving activities (e.g., dressing, bathing, and feeding) protect women in their early 80s from declining EPA. This seems plausible given that women are more likely to provide physical care for their husbands than men for their wives (Ingersoll-Dayton & Raschick, 2004) and that the young-old women in the present study were twice as likely to be living with another person than were their older counterparts (43% vs 20%). This is a hypothesis that will have to await future research, because the absence of caregiving measures in the present study prevented a test of whether day-to-day caregiving activities protect women from declining EPA.
Women were also less likely than men to be classified as inactive by a preponderance of quiescent episodes. This may reflect women’s greater familiarity with the indoor environment that has not been captured in prior studies of physical activity (Chipperfield, Newall, Chuchmach, Swift, & Haynes, in press). In light of the well-established finding that women are more functionally limited than men (Arber & Cooper, 1999), it is paradoxical that women were also less likely to be categorized as sedentary. Moreover, these findings suggest a reversal of the gender pattern found in prior research that has reported men to be more active than women.
Third, provocative findings emerged to demonstrate that EPA predicted 2-year mortality and to suggest that being sedentary is especially detrimental. The risk of dying was over 3 times higher among sedentary individuals, compared with their less sedentary counterparts, even after I statistically controlled for sedentary adults’ older age and their poorer physical, functional, and psychological status. That salutary effects can emerge from small gains in activity contradicts reports that benefits are restricted to higher intensity exercise (Manini et al., 2006). The present data are more consistent with findings showing that the greatest effects of physical activity emerge when one is comparing sedentary with moderately active individuals (Gillis & Hirdes, 1996), and that being active in any capacity is more beneficial than being inactive (Brach et al., 2004).
The present data also point to the possibility that being sedentary is a greater risk factor for men’s than women’s survival. In particular, whereas nearly 30% of sedentary men were deceased after 2 years, only 10% of sedentary women had died. This is particularly disturbing if older men have a greater risk of becoming sedentary. Although the small numbers of deaths in the present study underscore the need for caution about causal conclusions, a gender difference in the effect of being sedentary on mortality would be consistent with work showing that EPA relates to poorer health for men but not for women (Chipperfield et al., in press).
Despite the fact that statistical control was imposed to rule out alternative interpretations, a third variable may be operating, particularly if the present measures of physical, functional, or psychological health status failed to fully capture the more compromised status of sedentary, relative to their less sedentary counterparts. Of note, however, is that the supplemental analyses that examined alternate measures (i.e., self-rated physical health, basic activities of daily living, life satisfaction) continued to show that being sedentary predicted mortality. Nonetheless, future research should expand the net of alternative explanations to determine whether it is truly the lack of physical activity and not some other third variable such as helplessness or a “lost will to live” that accounts for why sedentary adults die sooner than their counterparts. In short, although this study could provide no evidence to suggest that the higher mortality among older sedentary adults was due to their poorer physical, functional, or psychological status, the present data do not definitively show that longevity was compromised by the lack of physical movement per se.
It is notable that, although being sedentary increased the odds of dying, being active did not lower those odds. It is premature, however, to conclude that being active has no protective effect on longevity, because the absence of an effect might be a function of the short follow-up period (2 years) that resulted in a small number of decedents. Moreover, even if boosting EPA beyond a certain threshold does not extend life, its value may be apparent in other ways, as suggested by the role of nonexercise activity thermogenesis in obesity and weight gain (Levine et al., 2005) and, more generally, by research showing a wide range of health benefits from physical activity (Wolinsky, Stump, & Clark, 1995).
Limitations and Strengths
Two potential measurement limitations must be considered. First, the classification of individuals as sedentary could be flawed if it simply captured those who were napping during the day. This, however, would be inconsistent with data showing no correlation between EPA and either reports of daytime napping (yes or no) or the number of naps (Newall et al., 2008). Moreover, contrary to an assumption that napping would be characterized by relatively lengthy durations of stillness, a supplemental analysis of raw data in the present study showed that the majority of sedentary participants (75%) had only brief durations of stillness lasting less than an average of 6 minutes. Thus, there is some confidence that the classification used to identify sedentary individuals was not due primarily to napping during the day.
A second potential measurement limitation involves the use of a single-day measure of EPA that may not provide a completely accurate account of general day-to-day physical activity levels. Although a single-day assessment has been shown to be reliable (Lambert, 2006), and it accurately quantifies a large amount of information from each respondent on that day, reliability might have been enhanced by extending the measurement period beyond 1 day. However, doing so might have eroded compliance or heightened discomfort that is due to sensitive skin among old adults, illustrating the compromise that must be balanced when one is choosing an assessment period (Westerterp, 1999). Nonetheless, to the extent that the EPA measure contained error, this would work against establishing its relation to mortality. In other words, had a more reliable measure been available, the magnitude of the relationship between EPA and mortality might have been larger than the one reported in this study.
In short, the use of an objective, quantifiable measure of EPA among very old adults (M = 85 years) permitted a unique analysis of just how active or inactive individuals are in very late life. The access to a representative sample allowed for the development of a descriptive profile that is likely to generalize to the broader population of community-dwelling older adults. However, it must be acknowledged that the present study, like most others, may have failed to capture the most functionally restricted individuals, therefore possibly underestimating the percentages of individuals who are sedentary in the overall population.
A major strength of the present study was the access to mortality data. Nonetheless, there are limitations to the short follow-up period (2 years) that resulted in few deaths. This makes it difficult to compare the present findings to other studies that have followed participants for 5 to 10 year (e.g., Bijnen et al., 1998; Lee et al., 2004). Nevertheless, the present findings are consistent with the existing body of research that suggests a linkage between physical activity and survival, providing a consensus in the field.
On the basis of the present findings, it is recommended that new trials be conducted to establish whether and why physical activity is associated with mortality. If such trials can show that minimizing inactivity saves lives, this would have compelling implications for the development of an unobtrusive, easy to implement intervention. Such an intervention would have advantages over those designed to promote exercise, because exercise can be impossible for some very old adults who are confined to their homes. Reducing sedentary time also likely bolsters quality of life, suggesting that the outcomes following such interventions should be extended to include quality as well as quantity of life. Reducing sedentary time may even delay admissions to personal care home and minimize the use of health services, resulting in savings for the health care system. In light of the national challenges facing the health care system, finding ways to enhance simple, everyday physical movement may have financial implications.
To conclude, although the present study showed that some older individuals continued to be physically active on a daily basis, some older adults in the present study were sedentary. The demonstration of a relationship between a sedentary life and mortality fits with a body of knowledge showing negative consequences of inactivity. Taken together, a simple message may be emerging from the accumulating evidence in the field: Older adults should be encouraged to avoid stillness and keep moving, even if only slowly.
Acknowledgments
This study was supported by a Canadian Institutes of Health Research (CIHR) grant (200609MOP-165097-SDA-CDAA-49702) and a CIHR Mid-Career Award in Aging. Greatly appreciated is the assistance provided by Loring Chuchmach, Nancy Newall, Audrey Swift, and Tara Haynes of the Laboratory for Aging and Health Research.
Footnotes
For 19 of the 22 chronic conditions in our study, it was possible to borrow the ranking scores from the Seriousness of Illness Ranking Scale (SIRS) as introduced by Wyler, Masuda, and Holmes (1968) and revised (SIRS-R) by Rosenberg, Hayes, and Peterson (1987). This could be done in the case of 9 conditions for which there was an exact match to a condition in the SIRS-R illness listing and for 10 conditions that had no exact match but had near matches to multiple SIRS-R illnesses. For each of these 10 conditions, an average ranking score was calculated from the SIRS-R illness rankings associated with the multiple near-matched illnesses. Finally, for 3 conditions in the present study that did not have either an exact or near match in the SIRS-R illness listing, two medical residents provided their best estimation as to where these 3 conditions ranked among the 19 ranked chronic conditions.
References
- Andersen LB, Schnohr P, Schroll M, Hein HO. All-cause mortality associated with physical activity during leisure time, work, sports, and cycling to work. Archives of Internal Medicine. 2000;160:1621–1628. doi: 10.1001/archinte.160.11.1621. [DOI] [PubMed] [Google Scholar]
- Arber S, Cooper H. Gender differences in health in later life: The new paradox? Social Science & Medicine. 1999;48:61–76. doi: 10.1016/s0277-9536(98)00289-5. [DOI] [PubMed] [Google Scholar]
- Armstrong GK, Morgan K. Stability and change in levels of habitual physical activity in later life. Age and Ageing. 1998;27:17–23. doi: 10.1093/ageing/27.suppl_3.17. [DOI] [PubMed] [Google Scholar]
- Bennet KM. Gender and longitudinal changes in physical activities in later life. Age and Ageing. 1998;27:24–28. doi: 10.1093/ageing/27.suppl_3.24. [DOI] [PubMed] [Google Scholar]
- Bijnen FC, Caspersen CJ, Feskens EJ, Saris WH, Mosterd WL, Kromhout D. Physical activity and 10-year mortality from cardiovascular diseases and all causes: The Zutphen Elderly Study. Archives of Internal Medicine. 1998;158:1499–1505. doi: 10.1001/archinte.158.14.1499. [DOI] [PubMed] [Google Scholar]
- Brach JS, Simonsick EM, Kritchevsky S, Yaffe K, Newman AB. Association between physical function and lifestyle activity and exercise in the Health, Aging and Body Composition Study. Journal of the American Geriatrics Society. 2004;52:502–509. doi: 10.1111/j.1532-5415.2004.52154.x. [DOI] [PubMed] [Google Scholar]
- Brodersen NH, Steptoe A, Boniface DR, Wardle J. Trends in physical activity and sedentary behaviour in adolescence: Ethnic and socioeconomic differences. British Journal of Sports Medicine. 2007;41:140–144. doi: 10.1136/bjsm.2006.031138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipperfield JG, Havens B, Doig W. Methods and description of the Aging in Manitoba Project: A 20-year longitudinal study. Canadian Journal on Aging. 1997;16:606–625. [Google Scholar]
- Chipperfield JG, Eaton WO. Reactivity and environmental stimulation as predictors of motor activity level in children. Personality and Individual Differences. 1992;13:591–601. [Google Scholar]
- Chipperfield JG, Newall NE, Chuchmach LP, Haynes TL, Ruthig JC, Perry RP, et al. Study of Everyday Physical Activity (EPA) 2003 in later life: Methods and description (Technical Report No. HLHPRI112) Winnipeg, Canada: University of Manitoba; Health, Leisure & Human Performance Research Institute; 2006. [Google Scholar]
- Chipperfield JG, Newall NE, Chuchmach LP, Swift AU, Haynes TL. Differential determinants of men’s and women’s everyday physical activity in later life. Journal of Gerontology: Social Sciences. 2008;63B doi: 10.1093/geronb/63.4.s211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipperfield JG, Perry RP. Primary- and secondary-control strategies in later life: Predicting hospital outcomes in men and women. Health Psychology. 2006;25:226–236. doi: 10.1037/0278-6133.25.2.226. [DOI] [PubMed] [Google Scholar]
- Chipperfield JG, Perry RP, Weiner B. Discrete emotions in later life. Journal of Gerontology: Psychological Sciences. 2003;58B:1–12. doi: 10.1093/geronb/58.1.p23. [DOI] [PubMed] [Google Scholar]
- Curtis J, White P, McPherson B. Age and physical activity among women and men: Findings from longitudinal national survey data. Journal of Aging and Physical Activity. 2000;8:1–19. [Google Scholar]
- Dishman RK, Washburn RA, Schoeller DA. Measurement of physical activity. Quest. 2001;53:295–309. [Google Scholar]
- Eaton WO. Temperament, development, and the five-factor model: Lessons from activity level. In: Halvorsen CF, Kohnstamm GF, editors. The developing structure of temperament and personality from infancy to adulthood. Hillsdale, NJ: Erlbaum; 1994. pp. 173–187. [Google Scholar]
- Eaton WO, Chipperfield JG, Singbeil CE. Birth order and activity level in children. Developmental Psychology. 1989;25:668–672. [Google Scholar]
- Eaton WO, Enns LR. Sex differences in human motor activity level. Psychological Bulletin. 1986;100:19–28. [PubMed] [Google Scholar]
- Esliger DW, Copeland JL, Barnes JD, Tremblay MS. Standardizing and optimizing the use of accelerometer data for free-living physical activity monitoring. Journal of Physical Activity and Health. 2005;3:366–383. [Google Scholar]
- Fukukawa Y, Nakashima C, Tsuboi S, Kozakai R, Doyo W, Niino N, et al. Age differences in the effect of physical activity on depressive symptoms. Psychology and Aging. 2004;19:346–351. doi: 10.1037/0882-7974.19.2.346. [DOI] [PubMed] [Google Scholar]
- Gillis KJ, Hirdes JP. Quality of life implications of health practices among older adults: Evidence from the 1991 Canadian General Social Survey. Canadian Journal on Aging. 1996;15:299–314. [Google Scholar]
- Hakim AA, Petrovich H, Burchfiel CM, Ross GW, Rodriguez BL, White LR, et al. Effects of walking on mortality among nonsmoking retired men. New England Journal of Medicine. 1998;338:94–99. doi: 10.1056/NEJM199801083380204. [DOI] [PubMed] [Google Scholar]
- Hoyt WT, Kerns MD. Magnitude and moderators of bias in observer ratings: A meta-analysis. Psychological Methods. 1999;4:403–424. [Google Scholar]
- Ingersoll-Dayton DB, Raschick M. Relationship between care-recipient behaviors and spousal caregiving stress. Gerontologist. 2004;44:318–327. doi: 10.1093/geront/44.3.318. [DOI] [PubMed] [Google Scholar]
- Kaplan GA, Seeman TE, Cohen RD, Knudsen LP, Guralnik J. Mortality among the elderly in the Alameda County Study: Behavioral and demographic risk factors. American Journal of Public Health. 1987;77:307–312. doi: 10.2105/ajph.77.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbe-Alexander TL, Lambert EV, Billetnikoff-Harkins J, Ekelund U. Comparison of two methods of measuring physical activity in South African older adults. Journal of Aging and Physical Activity. 2006;14:98–114. doi: 10.1123/japa.14.1.98. [DOI] [PubMed] [Google Scholar]
- Kushi LH, Fee RM, Folsom AR, Mink PJ, Anderson KE, Sellers TA. Physical activity and mortality in postmenopausal women. Journal of the American Medical Association. 1997;277:1287–1292. [PubMed] [Google Scholar]
- Lambert P. Master’s thesis. University of Manitoba; Winnipeg, Canada: University of Manitoba, Department of Community Health Sciences Dissertations; 2006. Physical activity and the oldest-old: A comparison of self-report and accelerometer readings. Thesis L173. [Google Scholar]
- Lee IM, Sesso HD, Oguma Y, Paffenbarger RS. The “Weekend Warrior” and risk of mortality. American Journal of Epidemiology. 2004;160:636–641. doi: 10.1093/aje/kwh274. [DOI] [PubMed] [Google Scholar]
- Levine JA, Lanningham-Foster LM, McCrady SK, Krizan AC, Olson LR, Kane PH, et al. Interindividual variation in posture allocation: Possible role in human obesity. Science. 2005;307(5709):584–586. doi: 10.1126/science.1106561. [DOI] [PubMed] [Google Scholar]
- Manini TM, Everhart JE, Patel KV, Schoeller DA, Colbert LH, Visser M, et al. Daily activity energy expenditure and mortality among older adults. Journal of the American Medical Association. 2006;296:171–179. doi: 10.1001/jama.296.2.171. [DOI] [PubMed] [Google Scholar]
- Mattiasson-Nilo I, Sonn U, Johannesson K, Gosman-Hedström G, Persson GB, Grimby G. Domestic activities and walking in the elderly: Evaluation from a 30-hour heart rate recording. Aging (Milano) 1990;2:191–198. doi: 10.1007/BF03323916. [DOI] [PubMed] [Google Scholar]
- Melanson ER, Freedson PS. Validity of the Computer Science and Applications, Inc. (CSA) activity monitor. Medicine and Science in Sports and Exercise. 1995;27:934–940. [PubMed] [Google Scholar]
- Mott S, Poole J, Kenrick M. Physical and chemical restraints in acute care: Their potential impact on the rehabilitation of older people. International Journal of Nursing Practice. 2005;11:95–101. doi: 10.1111/j.1440-172X.2005.00510.x. [DOI] [PubMed] [Google Scholar]
- MTI Health Services. Actigraph analysis software 3.0 user’s manual. 2000 [Online]. Available from the MTI Web site, http://www.mtiactigraph.com.
- Neugarten BL, Havighurst RJ, Tobin SS. The measurement of life satisfaction. Gerontology. 1961;16:134–143. doi: 10.1093/geronj/16.2.134. [DOI] [PubMed] [Google Scholar]
- Newall NE, Chipperfield JG, Chuchmach LP, Swift AU, Haynes TL, Menec VH. Successful Aging Study (SAS) and Everyday Physical Activity (EPA) Study 2006: Methods and description. 2008. Unpublished Technical Report. [Google Scholar]
- Rosenberg SJ, Hayes JR, Peterson RA. Revising the Seriousness of Illness rating scale: Modernization and re-standardization. International Journal of Psychiatry in Medicine. 1987;17:85–92. doi: 10.2190/jwmw-8q1u-71dj-an6e. [DOI] [PubMed] [Google Scholar]
- Sallis JF, Saelens BE. Assessment of physical activity by self-report: Status, limitations, and future directions. Research Quarterly for Exercise and Sport. 2000;71:S1–S14. [PubMed] [Google Scholar]
- Schoenborn CA. Health habits of U.S. adults, 1985: The “Alameda 7” revisited. Public Health Reports. 1986;101:571–580. [PMC free article] [PubMed] [Google Scholar]
- Schroll M. Physical activity in an ageing population. Scandinavian Journal of Medicine & Science in Sports. 2003;13:63–69. doi: 10.1034/j.1600-0838.2003.20226.x. [DOI] [PubMed] [Google Scholar]
- Sihvonen S, Rantanen T, Heikkinen E. Physical activity and survival in elderly people: A five-year follow-up study. Journal of Aging and Physical Activity. 1998;6:133–140. [Google Scholar]
- Slingerland AS, van Lenthe FJ, Jukema JW, Kamphuis CB, Looman C, Giskes K, et al. Aging, retirement, and changes in physical activity: Prospective cohort findings from the GLOBE study. American Journal of Epidemiology. 2007;15:1356–1363. doi: 10.1093/aje/kwm053. [DOI] [PubMed] [Google Scholar]
- Steele BG, Belza B, Cain K, Warms C, Coppersmith J, Howard J. Bodies in motion: Monitoring daily activity and exercise with motion sensors in people with chronic pulmonary disease. Journal of Rehabilitation Research and Development. 2003;40(Suppl 2):45–58. doi: 10.1682/jrrd.2003.10.0045. [DOI] [PubMed] [Google Scholar]
- Stessman J, Maaravi Y, Hammerman-Rozenberg R, Cohen A. The effects of physical activity on mortality in the Jerusalem 70-Year-Olds Longitudinal Study. Journal of the American Geriatric Society. 2000;48:499–504. doi: 10.1111/j.1532-5415.2000.tb04995.x. [DOI] [PubMed] [Google Scholar]
- Trost SG, Owen N, Bauman AE, Sallis JF, Brown W. Correlates of adults’ participation in physical activity: Review and update. Medicine and Science in Sports and Exercise. 2002;34:1996–2001. doi: 10.1097/00005768-200212000-00020. [DOI] [PubMed] [Google Scholar]
- Trost SG, Pate RR, Sallis JF, Freedson PS, Taylor WC, Dowda M, et al. Age and gender differences in objectively measured physical activity in youth. Medicine and Science in Sports and Exercise. 2002;34:350–355. doi: 10.1097/00005768-200202000-00025. [DOI] [PubMed] [Google Scholar]
- Welk GJ, Schaben JA, Morrow JR., Jr Reliability of accelerometry-based activity monitors: A generalizability study. Medicine and Science in Sports and Exercise. 2004;36:1637–1645. [PubMed] [Google Scholar]
- Westerterp KR. Physical activity assessment with accelerometers. International Journal of Obesity. 1999;23:S45–S49. doi: 10.1038/sj.ijo.0800883. [DOI] [PubMed] [Google Scholar]
- Wolinsky FD, Stump TE, Clark DO. Antecedents and consequences of physical activity and exercise among older adults. The Gerontologist. 1995;35:451–462. doi: 10.1093/geront/35.4.451. [DOI] [PubMed] [Google Scholar]
- Wyler AR, Masuda M, Holmes TH. Seriousness of Illness rating scale. Journal of Psychosomatic Research. 1968;11:363–374. doi: 10.1016/0022-3999(68)90033-0. [DOI] [PubMed] [Google Scholar]
- Zizzi S, Goodrich D, Wu Y, Parker L, Rye S, Pawar V, et al. Correlates of physical activity in a community sample of older adults in Appalachia. Journal of Aging and Physical Activity. 2006;14:423–438. doi: 10.1123/japa.14.4.423. [DOI] [PubMed] [Google Scholar]


