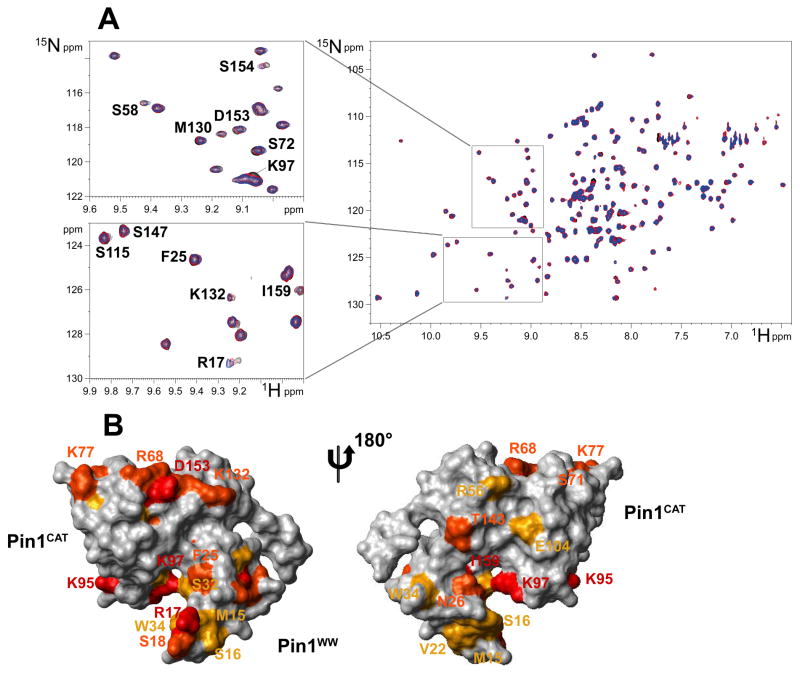
Figure 3. NMR spectroscopy mapping of surface residues in Pin1 that contact I-2.
(A) The 1H-15N HSQC spectrum of 15N-labeled Pin1 alone (black) overlaid with spectra after addition of 0.5 molar equivalent (red) and 1.0 molar equivalent (blue) of unlabelled I-2 showing the shifts of resonances, marked with single letter code and residue number. Zoom-in areas are enlarged to the left side for closer examination. (B) Mapping of the I-2 interaction sites on Pin1 structure (PDB ID: 1PIN). The isomerase domain (Pin1CAT) is to the upper left and the WW domain (Pin1ww) to the lower right side. The structure on the right is rotated 180° to show the reverse side. Residues with combined chemical shift variations (calculated as described in Methods) greater than 0.04 ppm are colored red, those in the 0.02 – 0.04 ppm range colored orange, and those in the 0.015 – 0.02 ppm range colored yellow.