Figure 5. Binding of I-2 variants to GST-Pin1.
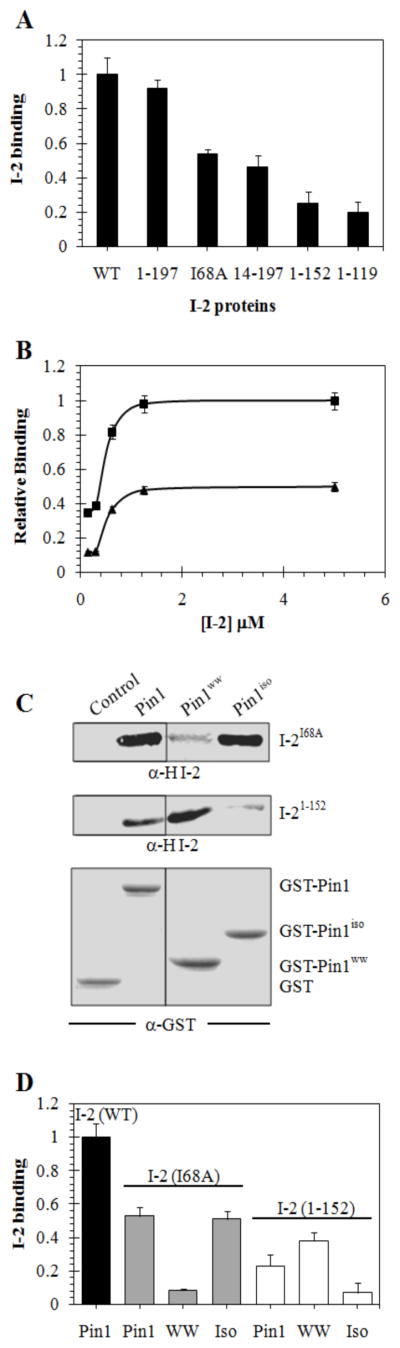
(A) Binding of wild type (WT) and truncated and substituted HI-2 to GST-Pin1 in pull down assay as described in Materials & Methods. The recovery of bound I-2 was quantitated using Odyssey software V1.2 by immunoblotting, with correction for amount of GST-Pin1 by simultaneous dual wavelength scanning of two different secondary antibodies on the same filter (Odyssey, Li-Cor Industries). Values from three independent experiments were normalized to WT, averaged, and plotted with S.D. (B) Dose response for binding of wt HI-2 (squares) and I-2(I68A) (triangles) to GST-Pin1 in pull down assay (n=3). Immunoblotting analyzed as described in (A) and data plotted with S.D. using Sigma plot 10.0 software. (C and D) Pull down assays for binding of 1.25 μM either HI-2(I68A) [I-2I68A] (upper panels in C, grey bars in D) or HI-2(1–152) [I-21–152] (center panels in C, open bars in D) with GST, GST-Pin1 [Pin1], GST-Pin1ww [Pin1ww, WW], or GST-Pin1iso [Pin1iso, Iso]. Immunoblotting for GST and fusion proteins is shown in C, lower panel. For control and normalization of other samples HI-2 wild type (WT) was pulled down with GST-Pin1. Data (n=3) analyzed as described in (A).
