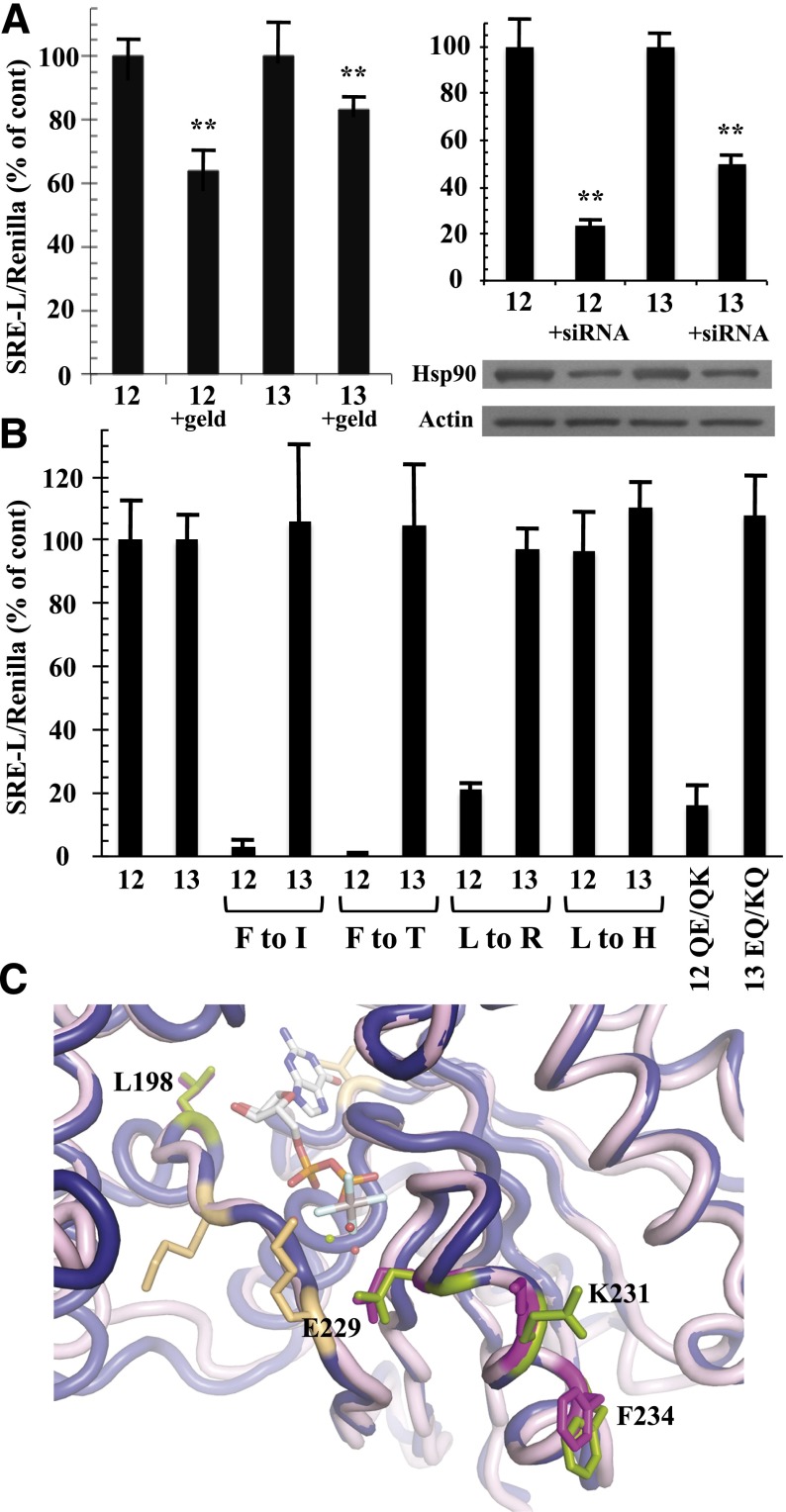
Fig. 5.
Differential effects of Hsp90 inhibition and SRE-uncoupling mutations in Gα12 and Gα13. (A) Effects of geldanamycin (left graph) and Hsp90-specific siRNA (right graph) on Gα12- and Gα13-mediated SRE activation are shown. HEK293 cells grown in 12-well plates were transfected with 0.2 µg of SRE-luciferase and 0.02 µg of pRL-TK, plus 1 µg of a constitutively active variant of either Gα12 (12) or Gα13 (13). For the left graph, cells were serum-starved for 24 hours, and then 1 µg/ml geldanamycin (geld) was added for 6 hours. For the right graph, siRNA specific to cytoplasmic Hsp90-α was cotransfected with the above plasmids as described in Materials and Methods. Luminometry assays were performed as described in Fig. 2, and each G12/13 control sample (geldanamycin absent, or siRNA absent) was set at 100%. For siRNA experiments, cell lysates were subjected to immunoblot analysis, using antibodies specific to Hsp90-α (Santa Cruz Biotechnology) and actin (clone C4; Millipore), and representative blots are shown. Results presented in each graph are the mean of three or more independent experiments, with error bars representing ± S.E.M. Effects of geldanamycin (left graph) and siRNA (right graph) were analyzed by one-way analysis of variance for significant difference in disruption of the Gα12 response compared with disruption of the Gα13 response (**P < 0.001). (B) HEK293 cells were transfected with SRE-luciferase and pRL-TK as described above plus 1 µg of each Gα plasmid. All constructs encoded the constitutively active form of the Gα protein (12 or 13), and substitutions of class-distinctive residues are indicated below the x-axis (e.g., F to I). Additional mutants were tested: QE/QK, Q232E/Q234K substitutions in constitutively active Gα12; EQ/KQ, E229Q/K231Q substitutions in constitutively active Gα13. Data are presented as mean ± range and are the result of three or more independent experiments per construct. (C) Rendering of Gα12 (PDB ID 1ZCA; Kreutz et al., 2006) is shown in blue cartoon and Gα13 (PDB ID 3AB3; Hajicek et al., 2011) in light pink cartoon with the bound nucleotide displayed as sticks and colored by atom type with carbons in white. For Gα13, side chains of Leu198, Glu229, Lys231, and Phe234 are displayed as magenta sticks and numbered. Corresponding Gα12 side chains of Leu201, Gln232, Gln234, and Phe237 are shown as green sticks and are not numbered in this diagram. Figure was rendered in The PyMOL Molecular Graphics System, Version 1.5.0.1 Schrödinger, LLC.