Abstract
Purpose
To describe changes and gender differences in the muscle-bone unit at different skeletal sites during pubertal development.
Methods
442 children aged 5-18 years were studied. Measurements of bone mineral content (BMC), lean mass (LM) and fat mass of the whole body (WB), legs, arms and lumbar spine were obtained from dual energy X-ray absorptiometry. Peripheral quantitative computed tomography was used to measure BMC of the radius diaphysis and cross-sectional muscle area (CSMA) of the mid-forearm. These measurements were used to describe differences between, and within, genders at each pubertal stage in BMC accrual relative to muscle, both before and after adjustment for height, regional fat and muscle at central and peripheral skeletal sites.
Results
In males there were significant increases in adjusted WB and leg BMC at the end of pubertal development. Unadjusted and adjusted lumbar spine BMC increased at the onset of, and at the end, of puberty. Radius BMC increased at most pubertal stages. In females, there were increases in unadjusted and adjusted whole body BMC at late puberty, in leg BMC at the onset of puberty, and at pubertal stage four. Unadjusted arm BMC increased at most pubertal stages; however, after adjustment an increase occurred at pubertal stage four. Both adjusted and unadjusted lumbar spine BMC increased at pubertal stage four. Unadjusted radius BMC increased at most pubertal stages. Females had greater BMC at all skeletal sites, compared to males, except at the radius, where adjusted BMC was greater in males at pubertal stage four.
Conclusions
Males and females accrue more BMC in relation to lean mass at multiple skeletal sites as puberty proceeds. Females accrue more BMC in relation to lean mass, in comparison to males, at most skeletal sites.
Keywords: muscle-bone unit; puberty; child; musculoskeletal development; dual energy X-ray absorptiometry; Tomography, X-ray computed
Introduction
During growth bones increase in size and the amount of mineral they contain, in addition to continually undergoing changes in internal and external geometry [1, 2]. These processes occur at different rates throughout the skeleton and take on a gender-specific course of development [3, 4]. Puberty is a time of rapid skeletal development and is crucial in determining lifelong bone health. Local and systemic factors which affect bone growth and development include growth factors, hormones and sex steroids [5, 6]. In recent years, the effect of muscle upon bone development has received considerable attention [7-12]. The greatest physiological loads placed upon the skeleton originate from muscle forces, which are generated by muscular contractions [13, 14]. According to the mechanostat theory, the loading imposed by muscles is thought to stimulate growing bones to adapt their geometry and mineral content to preserve, or increase, bone strength [13]. The mechanostat therefore provides a homeostasis in which muscle growth and increased forces do not cause mechanical failure of bones [13, 15]. In this way, muscle is considered to be a major determinant of the strength of growing bones [15], and muscle and bone work together as a conceptual operational unit which is known as the “muscle-bone unit” [10]. In children the muscle-bone unit may be assessed non-invasively using bone densitometry techniques, such as dual-energy X-ray absorptiometry (DXA) or peripheral quantitative computed tomography (pQCT) [10, 16]. Outcome measures of bone mineral content (BMC), lean (muscle) mass and cross-sectional muscle area (CSMA) can be used as surrogates of bone and muscle strength, respectively [8, 10-12, 17].
Studies in growing children using DXA or pQCT have shown the relationship between muscle mass and bone mass accumulation and have also indicated a gender-specific pattern of development, as females accumulate more bone in relation to muscle in comparison to males [7, 8, 10, 12, 16, 18]. Whilst such gender differences in the muscle-bone unit are well described, there have been no studies which have described both the gender-specific development of, and gender differences in, the muscle-bone unit at each stage of pubertal development at a number of skeletal sites. In addition, there have been no investigations of the muscle-bone unit using both DXA and pQCT. The benefit of using the two techniques is that the central and whole skeleton can be assessed with DXA, and pQCT gives a volumetric representation of a cross section though the bone which may provide greater insight into the evolution of the muscle bone unit during development without as much influence from linear growth.
It may be argued that gender differences in the muscle-bone unit are not because females accrue more bone in relation to muscle, but are due to gender differences in body composition. Females accrue more fat than lean mass in comparison to males; this may make females appear to accumulate more bone in relation to lean mass [19]. Additionally, the role of fat mass in skeletal growth has not been well defined, with a positive and a negative role in bone development being reported [20-22]; the relationship may be dependent upon gender and developmental stage [23]. To date, there are no studies which have assessed gender differences in the muscle-bone unit at each stage of pubertal development, after taking into account the differential accumulation of fat mass in males and females.
Therefore the primary objective of this study was to confirm and extend previous investigations of the muscle-bone unit during growth by combining technologies and investigating the contribution of body size and fat mass to the relationship [7, 8, 10, 11, 17]. This study aimed to: i) describe the cross-sectional development of the muscle-bone unit at both central and peripheral skeletal sites, in males and females with advancing puberty; ii) assess whether there were gender differences in the muscle-bone unit at a number of skeletal sites at each pubertal stage and iii) assess whether there were gender differences in the muscle-bone unit at a number of skeletal sites after adjusting for body size and fat mass.
Subjects and Methods
Subjects
442 (239 male) white Caucasian children aged between 5-18 years were studied. Subjects participated in the Manchester ‘Bone Density in Healthy Children’ study. The primary aim of this cross-sectional study was to produce reference data for the clinical interpretation of bone densitometry results in children [24, 25]. Recruitment for the study was through advertisement in educational institutions and general practitioner surgeries within the Greater Manchester region, United Kingdom. Detailed recruitment and relevant inclusion and exclusion criteria for the population have been described [24, 25]. Briefly, children of white Caucasian ethnic origin aged between 5-18 years were eligible for inclusion within the study. Subjects were excluded if they had i) conditions, or were taking medication, known to affect bone health, ii) systemic disease, iii) recurrent low trauma fractures, or iv) had sustained a fracture within the past 12 months. The study protocol was approved by the North West Multi-centre Research Ethics Committee (MREC 04/8/006) and was conducted in accordance with the Declaration of Helsinki. Informed verbal consent to participate in the study was obtained from all subjects in addition to written consent being obtained from subjects aged 16 years and older, or from parents or guardians of subjects who were younger than 16 years.
Anthropometric measurements
Pubertal stage was self-assessed in female subjects aged eight years and older and male subjects aged nine years and older, using a previously described method [26]. Females rated breast development as one of five pubertal stages (ranging from pre-pubertal [pubertal stage 1] to pubertal maturity [pubertal stage 5]), according to diagrams and descriptions based upon the Tanner criteria [27]. Males self-assessed left and right testicular volume (mL) by comparing the size of each testicle to a series of beads on a Prader orchidometer (Pharmacia and Upjohn, Uppsala, Sweden). Average left and right testicular volume was used to determine pubertal stage in males, whereby pubertal stage 1 = ≤ 3mL, pubertal stage 2 = 4-8 mL, pubertal stage 3 = 10-15 mL, pubertal stage 4 = 20mL and pubertal stage 5 = 25mL [28]. Weight (kg) was measured using Seca digital scales (Autoweigh Scales, United Kingdom) and standing height (cm) was measured using a wall-mounted stadiometer (Leicester Height Measure, Child Growth Foundation, United Kingdom). For pQCT measurements, the length of the non-dominant forearm (forearm length) (mm) was measured using a flexible tape measure (Sunlight Medical, Tel Aviv, Israel), and was defined as the distance from the ulna styloid process to the olecranon process.
Bone densitometry and body composition measurements
Bone densitometry assessments have been described in detail for the study population [24, 25]. In brief, the Hologic QDR 4500 Discovery DXA fan beam scanner (Bedford, MA, U.S.A.) was used to perform scans (fast array mode) of the whole body, from which were extracted parameters of whole and regional body composition, in accordance with manufacturer’s recommendations. BMC for lumbar spines (L1-4) were obtained from site specific scans. Whole body scans were analysed using the adult analysis algorithm, (software version 12.1) and lumbar spine scans using auto low density. Outcome measures used in the study were BMC (g) of the whole body and lumbar spine (L1-4). Additionally, BMC, lean (muscle) mass (LM) (g) and fat mass of regional body segments (?trunk, arms and legs) were derived from whole body scans (Figure 1). Two Stratec XCT-2000 pQCT scanners (Pforzheim, Germany) were used to scan the non-dominant radius diaphysis, at the site which corresponded to 50% of forearm length. One scanner, which was purchased in the early days of pQCT, has a narrower slice width (1.2mm) than the more modern scanner with a slice width of 2.0mm, which is now the routine slice width of such pQCT scanners. Consequently each section was taken at a voxel size of either 0.4 × 0.4 × 1.2 mm or 0.4mm × 0.4mm × 2mm. Scan speed was 25 mm/s−1. The resulting cross-sectional images were analysed using manufacturer’s software (version 5.5d). We tested differences between the two scanners by taking measurements of the European Forearm Phantom and healthy volunteers (n = 29), and the root mean square error between the scanners was compared to the precision error of the scanners. The differences between the scanners were less than the precision error for all the variables presented in this manuscript so no adjustments were judged to be necessary between scanners to pool the results. We verified this decision with the scanner manufacturer Stratec Medizintechnik GmbH, Pforzheim, Germany (Dr Johannes Willnecker - personal communication) and also independently with Dr Klaus Engelke, a CT expert from University of Erlangen (Germany).
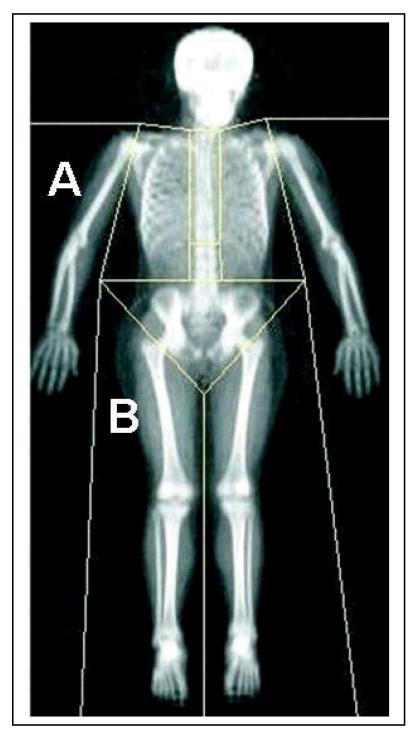
Figure 1.
Dual energy X-ray absorptiometry scan of the whole body, showing regional body segments (A = arm, B = leg).
[24]. Outcome measures used in this study were cortical BMC (mg/mm) and CSMA (mm2). Measurements of BMC were obtained using the CORTBD algorithm, separation mode 1, threshold 710 mg/cm3. CSMA measurements were obtained using the CALCBD algorithm, contour mode 3, peel mode 1, filter F03F05, threshold 40 mg/cm3. The muscle plus bone area was detected using the threshold of 40 mg/cm3 , to separate from the subcutaneous fat. Bone area (radius plus ulna) was then detected using a threshold of 280 mg/cm3 . By subtracting this from muscle plus bone area, cross-sectional muscle area is then calculated. Quality assurance on both scanners was performed on a daily basis, using standard manufacturers’ phantoms. The precision (coefficient of variation (%)) in adults in our Unit for bone and body composition outcome measures used in the study were 0.92% for whole body BMC, 0.56% for whole body LM, 1.75% for whole body fat mass and 3.72% for CSMA. The total estimated effective dose equivalent for all bone densitometry scans was 6.73 μSv [29, 30], which is less than 1.1 days of natural background radiation for the United Kingdom [31].
Statistical analyses
All data analysed were cross-sectional.
Statistical analyses were performed using SPSS (Chicago, IL, U.S.A), software version 15.0 and R version 2.8. Bone outcomes (whole body, leg, lumbar spine, arm and cortical BMC) were fitted to a linear (Analysis of covariance) model, with the corresponding muscle measurement (whole body, arm, leg lean mass and cross-sectional muscle area) as a linear covariate, so estimating BMC relative to muscle mass for the whole body, legs, arms, lumbar spine and radius. The effects of pubertal stage and gender were determined by adding appropriate covariates to this model, along with an interaction term between the two. A second model additionally adjusted for height and regional fat mass2. The significance of terms was determined by standard F-tests of the appropriate contrasts, including a test for differences from one pubertal stage to the next. The residuals from the fitted models were inspected visually to confirm the assumptions of the parametric model were reasonable.
The data were visualised as box and whisker plots for both the unadjusted muscle-bone unit (adjusted to the mean muscle content using the model including only muscle) and adjusted muscle-bone unit (adjusted to mean muscle content, height and fat content) measures, based on the two models described above. Within these plots, the box area contains the median (50th percentile) and interquartile range (25th - 75th percentile). The lower and upper whiskers correspond to the minimum and maximum data points. Data points which are greater than one and a half or three times the interquartile range from the upper or lower ends of the box are classified as outlying and extreme values, respectively [32]. Statistical significance was set at the 0.05 level, with no formal adjustment for multiple testing.
Results
The original study cohort consisted of 442 subjects; 442 had DXA whole body and lumbar spine scans and 392 had pQCT scans. From these, all DXA and pQCT data were excluded from 18 subjects, as they had either refused pubertal stage assessment or had abnormal bone densitometry results. The subjects with abnormal bone densitometry results underwent a thorough clinical assessment for underlying medical conditions by a metabolic bone disease paediatrician. There were further exclusions from the remaining study cohort (424 DXA, 374 pQCT) due to the presence of movement or metal artefacts, including naval piercings. The final total study population consisted of 422 subjects (226 males and 196 females). The total number of scans available from these subjects for determining muscle-bone unit measures were 415 (224 male) for whole body, 422 (226 male) for leg, 422 (228 male) for arm, 413 (222 male) for lumbar spine and 368 (198 male) for radius/mid-forearm (referred to as the radius). Table 1 shows the number of males and females at each pubertal stage, with corresponding ages.
Table 1.
Age of males and females at each pubertal stage (total n = 422).
| Male | Female | |||
|---|---|---|---|---|
| PS | n | Age (years) median (IQR) | n | Age (years) median (IQR) |
| 1 | 79 | 8.7 (6.8 - 10.3) | 76 | 8.5 (7.0 - 9.1) |
| 2 | 42 | 12.2 (11.7 - 12.8) | 18 | 10.9 (10.1 - 12.0) |
| 3 | 64 | 13.8 (12.4 - 15.5) | 38 | 12.8 (12.1 - 14.3) |
| 4 | 23 | 15.2 (13.3 - 16.1) | 28 | 15.2 (13.9 - 16.2) |
| 5 | 18 | 16.6 (15.2 - 17.2) | 36 | 17.1 (15.8 - 17.8) |
| TOTAL | 226 | 196 | ||
PS = Pubertal stage; IQR = Interquartile range (25th - 75th percentile)
Figures 2 - 6 show the changes in unadjusted and adjusted muscle-bone unit measurements for the whole body (Figure 2), legs (Figure 3), arms (Figure 4), lumbar spine (Figure 5) and radius (Figure 6), in males and females, with advancing pubertal stage. Tables 2 - 6 show mean unadjusted and adjusted muscle-bone unit measurements for the whole body (Table 2), legs (Table 3), arms (Table 4), lumbar spine (Table 5) and radius (Table 6), in males and females, at each pubertal stage. Significant differences between successive pubertal stages for each gender, and gender differences at each pubertal stage are indicated. Gender*pubertal stage interactions for the muscle-bone unit at each site are also shown.
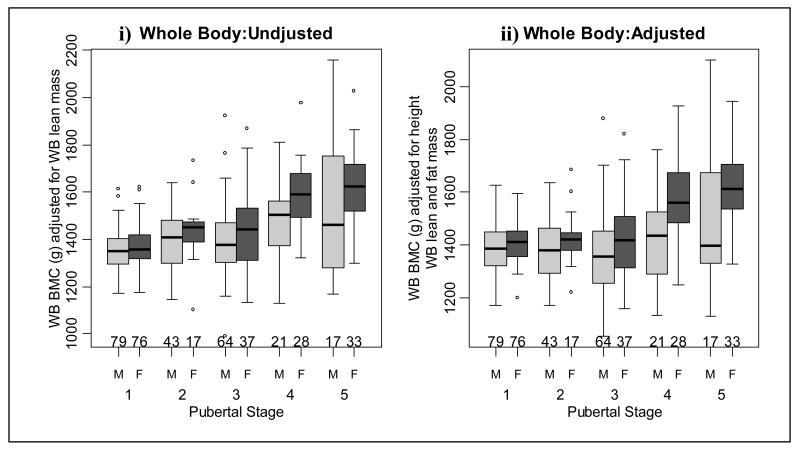
Figure 2.
Whole body (WB) bone mineral content (BMC) i) adjusted for WB lean mass and ii) adjusted for height, WB lean and fat mass in males and females at each pubertal stage.
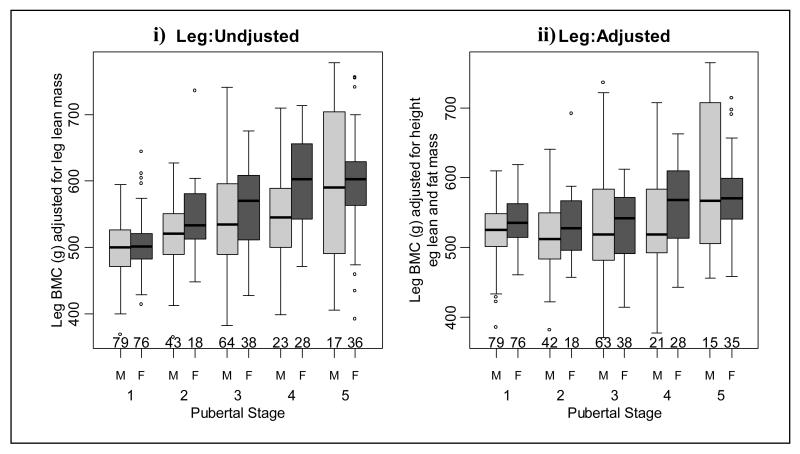
Figure 3.
Leg bone mineral content (BMC) i) adjusted for leg lean mass and ii) adjusted for height, leg lean and fat mass in males and females at each pubertal stage.
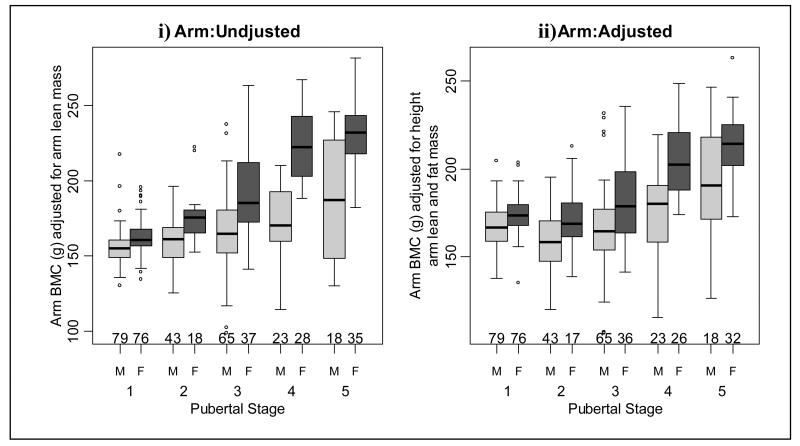
Figure 4.
Arm bone mineral content (BMC) i) adjusted for arm lean mass and ii) adjusted for height, arm lean and fat mass in males and females at each pubertal stage.
Figure 5.
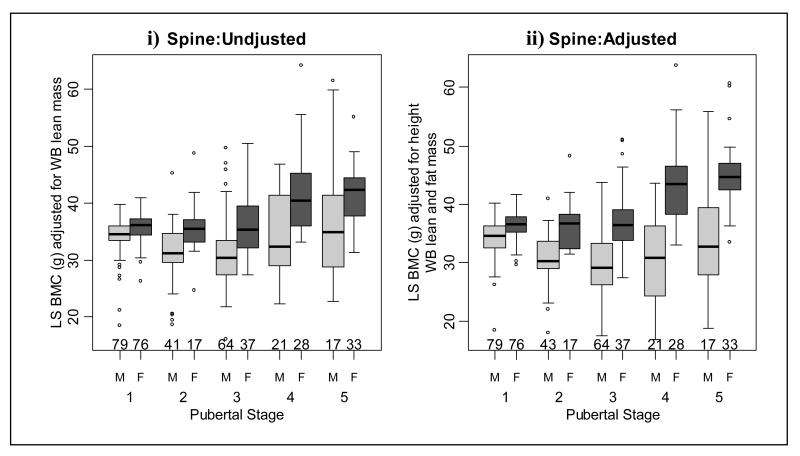
Lumbar spine (LS) bone mineral content (BMC) i) adjusted for whole body (WB) lean mass and ii) adjusted for height, WB lean and fat mass in males and females at each pubertal stage.
Figure 6.
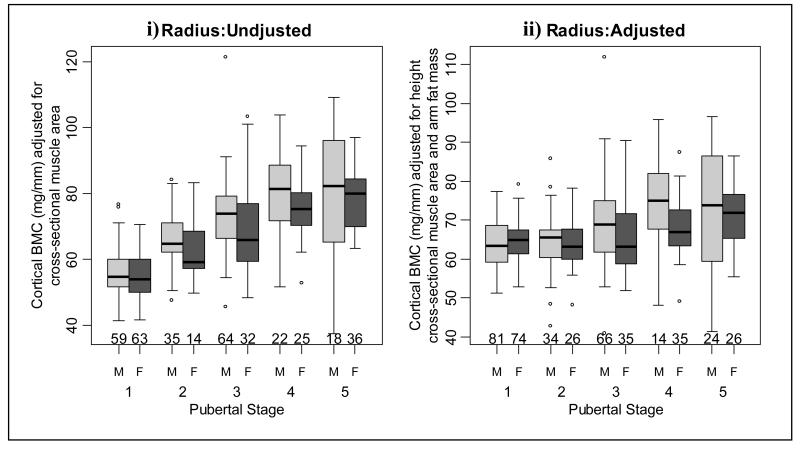
Radius cortical bone mineral content (BMC) i) adjusted for arm lean mass and ii) adjusted for height, arm lean and fat mass in males and females at each pubertal stage.
Table 2.
Whole body BMC adjusted for i) whole body lean mass and ii) height, whole body lean and fat mass in males and females at each pubertal stage. Differences between successive pubertal stages for each gender and also gender differences at each pubertal stage are indicated.
| Whole body BMC (g) adjusted for whole body lean mass (g) | Whole body BMC (g) adjusted for height, whole body lean and fat masses (g) | |||||||
|---|---|---|---|---|---|---|---|---|
| Males (mean (SE)) | Females (mean (SE)) | Mean Difference (SE) | P | Males (mean (SE)) | Females (mean (SE)) | Mean Difference (SE) | P | |
| Pubertal stage 1 | 1352.1 (19.1) | 1369.0 (20.9) | 16.9 (22.5) | 0.451 | 1383.1 (19.0) | 1409.1 (20.7) | 26.0 (21.3) | 0.224 |
| Pubertal stage 2 | 1390.6 (21.2) | 1438.4 (34.1) | 47.8 (40.1) | 0.233 | 1370.8 (20.2) | 1429.3 (32.6) | 58.5 (38.1) | 0.126 |
| Pubertal stage 3 | 1400.0 (20.2) | 1445.3 (22.8) | 45.3 (30.3) | 0.135 | 1373.0 (19.6) | 1420.1 (22.8) | 47.1 (29.9) | 0.116 |
| Pubertal stage 4 | 1464.2 (36.4) | 1557.3 (27.4)A | 119.3 (42.9) | 0.006 | 1413.6 (36.0) | 1583.5 (26.7)C | 143.7 (44.4) | 0.001 |
| Pubertal stage 5 | 1532.2 (42.3) | 1614.8 (25.9) | 82.6 (44.5) | 0.064 | 1506.0 (41.7)B | 1627.2 (26.9)D | 121.3 (47.8) | 0.012 |
| Muscle (per g) | - | - | - | <0.001 | - | - | 0 | <0.001 |
| Height (per cm) | - | - | - | - | - | - | 6.3 (1.1) | <0.001 |
| Fat (per g) | - | - | - | - | - | - | 0 | 0.001 |
| Interaction | - | - | - | 0.266 | - | - | - | 0.115 |
| Linear Interaction | - | - | - | 0.052 | - | - | - | 0.025 |
Significant difference compared to previous pubertal stage: p<0.001,
Significant difference compared to previous pubertal stage: p=0.034,
Significant difference compared to previous pubertal stage: p<0.001,
Significant difference compared to previous pubertal stage: p=0.043.
Table 3.
Leg BMC adjusted for i) whole body lean mass and ii) height, leg lean and fat mass in males and females at each pubertal stage. Differences between successive pubertal stages for each gender and also gender differences at each pubertal stage are indicated.
| Leg BMC (g) adjusted for leg lean mass (g) | Leg BMC (g) adjusted for height, leg lean and fat masses (g) | |||||||
|---|---|---|---|---|---|---|---|---|
| Males (mean (SE)) | Females (mean (SE)) | Mean Difference (SE) | P | Males (mean (SE)) | Females (mean (SE)) | Mean Difference (SE) | P | |
| Pubertal stage 1 | 496.1 (8.8) | 506.6 (9.6) | 10.4 (10.4) | 0.317 | 523.4 (8.4) | 537.2 (9.1) | 13.8 (9.4) | 0.141 |
| Pubertal stage 2 | 521.1 (9.8) | 534.7 (13.8)A | 22.1 (18.2) | 0.226 | 514.4 (8.8) | 543.2 (15.3) | 20.3 (16.5) | 0.217 |
| Pubertal stage 3 | 541.7 (9.3) | 559.0 (10.5) | 17.4 (13.9) | 0.212 | 531.1 (8.6) | 531.1 (9.9) | 0.1 (13.1) | 0.996 |
| Pubertal stage 4 | 548.2 (16.1) | 602.5 (12.3)B | 54.3 (19.5) | 0.006 | 537.6 (15.5) | 560.8 (12.2)D | 23.2 (19.7) | 0.239 |
| Pubertal stage 5 | 587.8 (19.2) | 599.5 (11.4) | 11.7 (20.3) | 0.566 | 591.4 (18.0)C | 573.2 (11.3) | −18.2 (20.7) | 0.378 |
| Muscle (per g) | - | - | 0.1 (0) | <0.001 | - | - | 0.0 (0.0) | <0.001 |
| Height (per cm) | - | - | - | - | - | - | 4.7 (0.5) | <0.001 |
| Fat (per g) | - | - | - | - | - | - | 0.0 (0.0) | 0.342 |
| Interaction | - | - | - | 0.349 | - | - | - | 0.359 |
| Linear Interaction | - | - | - | 0.361 | - | - | - | 0.276 |
Significant difference compared to previous pubertal stage: p=0.035,
Significant difference compared to previous pubertal stage: p=0.007 ,
Significant difference compared to previous pubertal stage: p=0.004,
Significant difference compared to previous pubertal stage: p=0.042.
Table 4.
Arm BMC adjusted for i) arm lean mass and ii) height, arm lean and fat mass in males and females at each pubertal stage. Differences between successive pubertal stages for each gender and also gender differences at each pubertal stage are indicated.
| Arm BMC (g) adjusted for arm lean mass (g) | Arm BMC (g) adjusted for height, arm lean and fat masses (g) | |||||||
|---|---|---|---|---|---|---|---|---|
| Males (mean (SE)) | Females (mean (SE)) | Mean Difference (SE) | P | Males (mean (SE)) | Females (mean (SE)) | Mean Difference (SE) | P | |
| Pubertal stage 1 | 155.7 (2.8) | 162.4 (3.0) | 6.7 (3.4) | 0.051 | 166.8 (2.8) | 173.4 (3.0) | 6.6 (3.1) | 0.035 |
| Pubertal stage 2 | 160.9 (3.2) | 176.1 (5.1)B | 15.3 (6.0) | 0.011 | 158.3 (3.0)E | 172.7 (4.6) | 14.4 (5.5) | 0.009 |
| Pubertal stage 3 | 167.8 (3.1) | 192.2 (3.5)C | 24.4 (4.7) | <0.001 | 165.4 (2.8) | 182.6 (3.3) | 17.2 (4.3) | <0.001 |
| Pubertal stage 4 | 172.6 (5.5) | 222.7 (4.0)D | 50.1 (6.8) | <0.001 | 174.2 (5.1) | 204.6 (4.1)G | 30.4 (6.6) | <0.001 |
| Pubertal stage 5 | 188.5 (6.4)A | 230.3 (3.6) | 41.8 (7.0) | <0.001 | 190.9 (5.9)F | 213.0 (3.8) | 22.1 (6.9) | 0.001 |
| Muscle (per g) | - | - | 0.1 (0.00) | <0.001 | - | - | 0.0 (0.0) | <0.001 |
| Height (per cm) | - | - | - | - | - | - | 1.1 ( 0.1) | <0.001 |
| Fat (per g) | - | - | - | - | - | - | 0.0 (0.0) | <0.001 |
| Interaction | - | - | - | <0.001 | - | - | - | 0.017 |
| Linear Interaction | - | - | - | <0.001 | - | - | - | 0.003 |
Significant difference compared to previous pubertal stage: p=0.019,
Significant difference compared to previous pubertal stage: p=0.015,
Significant difference compared to previous pubertal stage: p=0.009,
Significant difference compared to previous pubertal stage: p<0.001,
Significant difference compared to previous pubertal stage: p=0.041,
Significant difference compared to previous pubertal stage: p=0.007,
Significant difference compared to previous pubertal stage: p<0.001.
Table 5.
Lumbar spine BMC adjusted for i) whole body lean mass and ii) height, whole body lean and fat mass in males and females at each pubertal stage. Differences between successive pubertal stages for each gender and also gender differences at each pubertal stage are indicated.
| Lumbar spine BMC (g) adjusted for whole body lean mass (g) | Lumbar spine BMC (g) adjusted for height, whole body lean and fat masses (g) | |||||||
|---|---|---|---|---|---|---|---|---|
| Males (mean (SE)) | Females (mean (SE)) | Mean Difference (SE) | P | Males (mean (SE)) | Females (mean (SE)) | Mean Difference (SE) | P | |
| Pubertal stage 1 | 34.1 (0.8) | 35.8 (0.9) | 1.8 (0.9) | 0.056 | 34.1 (0.8) | 36.5 (0.8) | 2.4 (0.8) | 0.004 |
| Pubertal stage 2 | 31.1 (0.9)A | 35.6 (1.4) | 4.5 (1.6) | 0.006 | 30.6 (0.8)D | 36.5 (1.3) | 5.9 (1.5) | <0.001 |
| Pubertal stage 3 | 31.3 (0.8) | 36.5 (0.9) | 5.2 (1.2) | <0.001 | 29.8 (0.8) | 37.3 (0.9) | 7.5 (1.2) | <0.001 |
| Pubertal stage 4 | 33.9 (1.5) | 41.6 (1.1)C | 7.8 (1.7) | <0.001 | 30.1 (1.4) | 43.3 (1.1)F | 13.1 (1.8) | <0.001 |
| Pubertal stage 5 | 38.1 (1.7)B | 41.8 (1.1) | 3.7 (1.8) | 0.040 | 34.4 (1.6)E | 45.0 (1.1) | 10.7 (1.9) | <0.001 |
| Muscle (per g) | - | - | 0.0 (0.0) | <0.001 | - | - | 0.0 (0.0) | <0.001 |
| Height (per cm) | - | - | - | - | - | - | 0.0 (0.0) | 0.288 |
| Fat (per g) | - | - | - | - | - | - | 0.0 (0.0) | <0.001 |
| Interaction | - | - | - | 0.022 | - | - | - | <0.001 |
| Linear Interaction | - | - | - | 0.018 | - | - | - | <0.001 |
Significant difference compared to previous pubertal stage: p=0.011,
Significant difference compared to previous pubertal stage: p=0.024,
Significant difference compared to previous pubertal stage: p<0.001,
Significant difference compared to previous pubertal stage: p=0.002,
Significant difference compared to previous pubertal stage: p=0.014,
Significant difference compared to previous pubertal stage: p<0.001.
Table 6.
Radius (cortical) BMC adjusted for i) arm lean mass and ii) height, arm lean and fat mass in males and females at each pubertal stage. Differences between successive pubertal stages for each gender and also gender differences at each pubertal stage are indicated.
| Radius BMC (mg/mm) adjusted for arm lean mass (g) | Radius BMC (mg/mm) adjusted for height, arm lean and fat masses (g) | |||||||
|---|---|---|---|---|---|---|---|---|
| Males (mean (SE)) | Females (mean (SE)) | Mean Difference (SE) | P | Males (mean (SE)) | Females (mean (SE)) | Mean Difference (SE) | P | |
| Pubertal stage 1 | 56.0 (1.5) | 55.0 (1.5) | −1.0 ( 1.9 | 0.583 | 63.9 (1.5) | 64.8 (1.6) | 1.0 (1.6) | 0.546 |
| Pubertal stage 2 | 65.9 (1.8)A | 62.0 (2.8)D | −3.9 (3.3) | 0.241 | 64.8 (1.5)G | 63.3 (2.4) | −1.5 (2.9) | 0.605 |
| Pubertal stage 3 | 73.3 (1.4)B | 68.9 (1.9)E | −4.4 (2.4) | 0.066 | 69.3 (1.3)H | 65.5 (1.7) | −3.8 (2.1) | 0.069 |
| Pubertal stage 4 | 79.9 (2.6)C | 74.9 (2.1)F | −5.0 (3.4) | 0.137 | 74.1 (2.3) | 68.0 (1.9) | −6.1 (3.0) | 0.043 |
| Pubertal stage 5 | 79.7 (2.9) | 78.2 (1.8) | −1.5 (3.3) | 0.656 | 72.0 (2.6) | 71.0 (1.7) | −1.0 (3.0) | 0.746 |
| Muscle (per cm2) | - | - | 0.0 (0.0) | <0.001 | - | - | 0.0 (0.0) | <0.001 |
| Height (per cm) | - | - | - | - | - | - | 0.6 (0.1) | <0.001 |
| Fat (per g) | - | - | - | - | - | - | 0.0 (0.0) | 0.366 |
| Interaction | - | - | - | 0.717 | - | - | - | 0.201 |
| Linear Interaction | - | - | - | 0.559 | - | - | - | 0.144 |
Significant difference compared to previous pubertal stage: p<0.001,
Significant difference compared to previous pubertal stage: p=0.002,
Significant difference compared to previous pubertal stage: p=0.014,
Significant difference compared to previous pubertal stage: p=0.024,
Significant difference compared to previous pubertal stage: p=0.041,
Significant difference compared to previous pubertal stage: p=0.032,
Significant difference compared to previous pubertal stage: p=0.030,
Significant difference compared to previous pubertal stage: p=0.043
Males
Unadjusted whole body (Figure 2i and table 2) and leg BMC (Figure 3i and table 3) values were similar when successive pubertal stage groups were compared. However, both adjusted whole body and leg BMC increased at pubertal stage 5 (Figures 2ii and 3ii and tables 2 and 3, respectively). Unadjusted arm BMC increased at pubertal stage 5 and adjusted arm BMC increased at pubertal stages 2 and 5 (Figure 4 and table 4). There were significant increases in adjusted and unadjusted lumbar spine BMC at pubertal stages 2 and 5 (Figure 5 and table 5). As shown in Figure 6 and Table 6, unadjusted radius BMC increased at pubertal stages 2, 3 and 4, and adjusted radius BMC increased at pubertal stages 2 and 3.
Females
When consecutive pubertal stages were compared, unadjusted whole body BMC increased at pubertal stage 4 (Figure 2i and table 2). However, after adjustment there was an increase in whole body BMC at pubertal stages 4 and 5 (Figure 2ii and table 2). Unadjusted leg BMC increased at pubertal stages 2 and 4 (Figure 3i and table 3) and there was an increase in adjusted leg BMC at pubertal stage 4 (Figure 3ii and table 3). As shown in Figure 4 and table 4, unadjusted arm BMC increased at pubertal stages 2-4, whilst adjusted arm BMC increased at pubertal stage 4 only. Both adjusted and unadjusted lumbar spine BMC increased at pubertal stage 4 (Figure 5 and table 5). Unadjusted radius BMC increased between pubertal stages 2-4 (Figure 6i and table 6); however adjusted radius BMC values were similar when successive pubertal stages were compared (Figure 6ii and table 6).
Gender differences in muscle-bone unit measurements
Tables 2-6 show unadjusted and height and regional fat mass adjusted BMC in females compared to males for the whole body (table 2), legs (table 3), arms (table 4), lumbar spine (table 5) and radius (table 6).
There were significant interactions between gender and pubertal stage on unadjusted arm and lumbar spine BMC (tables 4 and 5) and height- and fat-adjusted whole body, arm and lumbar spine BMC (tables 2, 4 and 5, respectively). This indicates the effect of puberty on unadjusted and adjusted muscle-bone unit measurements was different for males and females. As shown in tables 2 and 3, females had significantly higher unadjusted whole body and leg BMC, compared to males, at pubertal stage 4. After adjustment for height and regional fat mass, these gender differences occurred for WB BMC at pubertal stages 4 and 5. Unadjusted arm BMC (table 4) and unadjusted lumbar spine BMC (table 5) were significantly greater in females at pubertal stages 2-5. After adjusting for height and arm fat mass, females had significantly greater arm and lumbar spine BMC at all pubertal stages, in comparison to males (tables 4 and 5). As shown in table 6, unadjusted radius BMC values were similar in males; however adjusted radius BMC was greater in males at pubertal stage 4.
Discussion
This study describes changes, and gender-differences, in the muscle-bone unit of the whole body and at peripheral and central skeletal sites at each stage of pubertal development before and after adjusting for height and regional fat mass.
The current study shows that both males and females tend to accumulate relatively greater amounts of BMC in comparison to muscle as puberty proceeds. A gender-specific pattern of development in the muscle bone unit was also shown. These results are in accordance with others who have shown a gender specific age-related increases in the muscle-bone unit at whole body [8, 12, 16, 17] and proximal radius [10, 18] sites. However, our data showed radius BMC increased in males and in females during puberty, and was greater in males at pubertal stage four, a finding not reported previously. Others have shown little change in the muscle bone unit at the radius with advancing puberty in males [10, 18]. We cannot explain the gender differences between DXA and pQCT results in the arm and radius respectively, in that males have more radius BMC after adjusting for height, cross-sectional muscle area and fat mass, compared to females. Given that pQCT measures a thin cross-section of bone rather than a projected area (DXA), it is feasible technical differences might explain the altered morphology between groups. Additionally, these different results may be due to the assessment of the muscle-bone unit by pQCT at different sites (midshaft (50%) versus proximal (66%) radius), or perhaps population differences between geographical locations [33, 34]. It is also possible that important covariates, such as socioeconomic status or physical activity levels, might explain such gender differences, more than height and fat mass. This interesting and surprising observation requires further study and clarification concerning the sexual dimorphism of bone geometry.
In the current study, there was no gender/pubertal stage interaction in unadjusted and adjusted leg BMC. This suggests that at weight-bearing skeletal sites males and females adapt to mechanical challenges in a similar way [35]. However, results from the current study contrast with those obtained by Macdonald et al. [11], who showed, after an interval of 20 months, the ratio of cortical area to CSMA at the tibia as measured by pQCT, decreased in both females and males. The differences between studies may be due to different measurement techniques (DXA vs. pQCT), sites and outcome measures, classification of puberty, longitudinal versus cross-sectional study design and cohort sizes.
The tempo of growth in height, bone and body composition follows a sequence of events, whereby peak height velocity (representing longitudinal bone growth) occurs first and is followed by peak LM, BMC and fat mass velocities [9, 36]. It is feasible that the gender-specific increases found in the muscle-bone unit in the current study may reflect this sequence of events during growth. It may be hypothesised that at the onset of puberty there is an increase in bone length and subsequent increase in BMC, with an accompanying increase in muscle length rather than width [37]. The continued growth of bone and muscle throughout puberty presents a continuous challenge to the stability of the growing bone [38, 39] which adapts to these by changing geometry and increasing mineral content to maintain bone strength [13, 15, 38, 40, 41]. This would account for further increases in the muscle-bone unit as puberty proceeds in males and females in our study, as measured by DXA and pQCT.
Whilst males have more bone and muscle in comparison to females [37, 42-51], our data show females tended to accumulate more BMC in relation to LM and confirms and extends the results of previous studies of the whole body [7, 8, 12, 16, 17], lumbar spine [12] and radius [10, 18]. Our data are novel in that they show these gender differences are independent of body size and differential fat mass accumulation in females.
Muscle development plateaus at an earlier age in females, and females accrue greater amounts of fat mass in relation to muscle. This would make females appear to accumulate more BMC in relation to LM, in comparison to males [19]. Ferretti et al. [17] showed that after adjusting for whole body fat mass, the ratio of whole body BMC to lean mass was still greater in pubertal females, but of lesser magnitude compared to unadjusted gender differences. This finding is in contrast to results from the current study and may reflect the different approaches used to adjust for fat mass, and that we have additionally adjusted for height.
The hypothesis that oestrogen is responsible for the gender differences in muscle bone unit is supported by results in the current study, as significant increases in adjusted BMC in females typically occurred at pubertal stage 4. This is the time when oestrogen levels peak and menarche occurs in females [52, 53]. The mechanism is thought to be through oestrogen lowering the mechanostat remodelling threshold at the endosteal surface of long bones, causing the endosteal surface to become more sensitive to strains induced by mechanical loading and causing an increase in bone deposition at the endosteal surface [16, 40, 54]. Schoenau et al. [18] showed that whilst there was a similar relationship between bone area and CSMA in males and females, there was a different relationship between medullary area and CSMA. These data suggest that gender differences in the ratio of BMC:CSMA occur via the relatively greater accumulation of bone on the endosteal surface in females, compared to males [18]. In support of this view, some [3, 55, 56] but not all [57] studies have reported significant decreases in medullary area of the metacarpal, tibia and mid-shaft femur in females at this time. Also, females with Turner’s syndrome, who are typically oestrogen deficient, had less BMC:CSMA due to increased medullary area at the proximal radius, in comparison to oestrogen replete controls [58]. As healthy females appear to accrue additional bone at the time when they potentially have the capacity to reproduce it is possible this extra bone may act as a reservoir for the potential mineral demands of pregnancy and lactation [16, 18].
There are some limitations to the current study. Whilst the data lend support to the theory that there is a relationship between muscle and bone based upon the mechanostat theory, the results may also be due to a commonality of growth between bone and muscle [59]. Moreover, this relationship may be regulated by genetic factors [60], in addition to local and systemic factors such as exercise and diet which were not assessed in this study [5, 6]. DXA makes certain assumptions, and this may vary between DXA manufacturers, when calculating bone mineral and body composition from X-ray beams of only two (rather than three) energies, so there may be some co-linearity of data using DXA from the same manufacturer, but this will apply to all such similar research studies. Indices used to investigate the muscle-bone unit, as derived from bone densitometry, are simply non-invasive surrogates of bone and muscle strength [61]. The current study may lack sufficient statistical power to detect differences between, and within, genders due to the small number of subject numbers at some pubertal stages. However, if pubertal stage groups had been combined into pre-, peri- and post-pubertal groups this would have resulted in a loss of information regarding changes in muscle-bone unit indices at important developmental stages, such as at the onset of puberty (pubertal stage 2) and at menarche (pubertal stage 4) in females.
The study is of cross-sectional study design, so we are unable to accurately characterise true changes in the muscle-bone unit over time in individual subjects, particularly the velocity and acceleration of these parameters during pubertal development. Whilst longitudinal studies can describe such changes, these require a considerable amount of time, dedication from participants and are more costly to undertake, in comparison to cross-sectional studies. Additionly, the study population for these analyses did not extend beyond 18 years of age. Whilst we have probably captured the end of the growth period in females, growth in males can continue beyond this age range [42, 47, 51], so there may be further increases in BMC and LM in males not captured in the current study [16, 17].
In conclusion, this study confirms that there are increases in surrogates of bone and muscle strength in both genders during puberty, supporting the mechanostat hypothesis. There is also a gender-specific pattern of development in the muscle-bone unit which results in females accruing relatively greater amounts of BMC per unit of lean mass, in comparison to males, at most skeletal sites. These gender differences appear to be independent of skeletal location, body size or differential fat mass accumulation.
Acknowledgements
The authors would like to thank the research subjects and their families for taking part in this study and the schools where recruitment was undertaken. Thanks are also due to Mr Mike Machin, database manager, for preparation of the bone density databases used. We gratefully acknowledge financial support from the National Osteoporosis Society (Camerton, Bath, United Kingdom) who awarded Rebecca Ashby a Linda Edwards Memorial PhD Studentship in 2003 and awarded a project grant for the initial part of the study, and the Central Manchester University Hospitals NHS Foundation Trust Research Endowment Fund, which funded the study.
Footnotes
Regional fat mass refers to fat mass of either the whole body, arms or legs. Therefore, whole body BMC and lumbar spine BMC were adjusted for whole body fat mass, arm and radius BMC for arm fat mass and leg BMC for leg fat mass.
All authors have nothing to disclose.
References
- 1.Parfitt AM. The two faces of growth: benefits and risks to bone integrity. Osteoporos Int. 1994;4:382–398. doi: 10.1007/BF01622201. [DOI] [PubMed] [Google Scholar]
- 2.Parfitt AM, Travers R, Rauch F, Glorieux FH. Structural and cellular changes during bone growth in healthy children. Bone. 2000;27:487–494. doi: 10.1016/s8756-3282(00)00353-7. [DOI] [PubMed] [Google Scholar]
- 3.Bass S, Delmas PD, Pearce G, Hendrich E, Tabensky A, Seeman E. The differing tempo of growth in bone size, mass, and density in girls is region-specific. J Clin Invest. 1999;104:795–804. doi: 10.1172/JCI7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradney M, Karlsson MK, Duan Y, Stuckey S, Bass S, Seeman E. Heterogeneity in the growth of the axial and appendicular skeleton in boys: Implications for the pathogenesis of bone fragility in men. J Bone Miner Res. 2000;15:1871–1878. doi: 10.1359/jbmr.2000.15.10.1871. [DOI] [PubMed] [Google Scholar]
- 5.Lian JB, Stein GS, Canalis E, Gehron Robey P, Boskey AL. Bone formation: osteoblast lineage cells, growth factors, matrix proteins and the mineralization process. In: Favus MJ, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. Lippincott, Williams and Wilkins; Philadelphia: 1999. pp. 14–29. [Google Scholar]
- 6.Mundy GR, Chen D, Oyajobi BO. Bone remodeling. In: Favus MJ, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. Lippincott, Williams and Wilkins; Philadelphia: 2003. pp. 46–58. [Google Scholar]
- 7.Crabtree NJ, Kibirige MS, Fordham JN, Banks LM, Muntoni F, Chinn D, Boivin CM, Shaw NJ. The relationship between lean body mass and bone mineral content in paediatric health and disease. Bone. 2004;35:965–972. doi: 10.1016/j.bone.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Hogler W, Briody J, Woodhead HJ, Chan A, Cowell CT. Importance of lean mass in the interpretation of total body densitometry in children and adolescents. J Pediatr. 2003;143:81–88. doi: 10.1016/S0022-3476(03)00187-2. [DOI] [PubMed] [Google Scholar]
- 9.Rauch F, Bailey DA, Baxter-Jones A, Mirwald R, Faulkner R. The ‘muscle-bone unit’ during the pubertal growth spurt. Bone. 2004;34:771–775. doi: 10.1016/j.bone.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Schoenau E, Neu CM, Beck B, Manz F, Rauch F. Bone mineral content per muscle cross-sectional area as an index of the functional muscle-bone unit. J Bone Miner Res. 2002;17:1095–1101. doi: 10.1359/jbmr.2002.17.6.1095. [DOI] [PubMed] [Google Scholar]
- 11.Macdonald HM, Kontulainen SA, MacKelvie-O’Brien KJ, Petit MA, Janssen P, Khan KM, McKay HA. Maturity- and sex-related changes in tibial bone geometry, strength and bone-muscle strength indices during growth: A 20-month pQCT study. Bone. 2005;36:1003–1011. doi: 10.1016/j.bone.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Pludowski P, Matusik H, Olszaniecka M, Lebiedowski M, Lorenc RS. Reference values for the indicators of skeletal and muscular status of healthy Polish children. J Clin Densitom. 2005;8:164–177. doi: 10.1385/jcd:8:2:164. [DOI] [PubMed] [Google Scholar]
- 13.Frost HM. Bone “mass” and the “mechanostat”: a proposal. Anat Rec. 1987;219:1–9. doi: 10.1002/ar.1092190104. [DOI] [PubMed] [Google Scholar]
- 14.Frost HM. Bone’s mechanostat: a 2003 update. Anat Rec A Discov Mol Cell Evol Biol. 2003;275:1081–1101. doi: 10.1002/ar.a.10119. [DOI] [PubMed] [Google Scholar]
- 15.Frost HM, Schoenau E. The “muscle-bone unit” in children and adolescents: a 2000 overview. J Pediatr Endocrinol Metab. 2000;13:571–590. doi: 10.1515/jpem.2000.13.6.571. [DOI] [PubMed] [Google Scholar]
- 16.Schiessl H, Frost HM, Jee WS. Estrogen and bone-muscle strength and mass relationships. Bone. 1998;22:1–6. doi: 10.1016/s8756-3282(97)00223-8. [DOI] [PubMed] [Google Scholar]
- 17.Ferretti JL, Capozza RF, Cointry GR, Garcia SL, Plotkin H, Alvarez Filgueira ML, Zanchetta JR. Gender-related differences in the relationship between densitometric values of whole-body bone mineral content and lean body mass in humans between 2 and 87 years of age. Bone. 1998;22:683–690. doi: 10.1016/s8756-3282(98)00046-5. [DOI] [PubMed] [Google Scholar]
- 18.Schoenau E, Neu CM, Mokov E, Wassmer G, Manz F. Influence of puberty on muscle area and cortical bone area of the forearm in boys and girls. J Clin Endocrinol Metab. 2000;85:1095–1098. doi: 10.1210/jcem.85.3.6451. [DOI] [PubMed] [Google Scholar]
- 19.Tothill P, Hannan WJ. Bone mineral and soft tissue measurements by dual-energy X-ray absorptiometry during growth. Bone. 2002;31:492–496. doi: 10.1016/s8756-3282(02)00854-2. [DOI] [PubMed] [Google Scholar]
- 20.Clark EM, Ness AR, Tobias JH. Adipose tissue stimulates bone growth in prepubertal children. J Clin Endocrinol Metab. 2006;91:2534–2541. doi: 10.1210/jc.2006-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janicka A, Wren TA, Sanchez MM, Dorey F, Kim PS, Mittelman SD, Gilsanz V. Fat mass is not beneficial to bone in adolescents and young adults. J Clin Endocrinol Metab. 2007;92:143–147. doi: 10.1210/jc.2006-0794. [DOI] [PubMed] [Google Scholar]
- 22.Weiler HA, Janzen L, Green K, Grabowski J, Seshia MM, Yuen KC. Percent body fat and bone mass in healthy Canadian females 10 to 19 years of age. Bone. 2000;27:203–207. doi: 10.1016/s8756-3282(00)00314-8. [DOI] [PubMed] [Google Scholar]
- 23.Ackerman A, Thornton JC, Wang J, Pierson RN, Jr., Horlick M. Sex difference in the effect of puberty on the relationship between fat mass and bone mass in 926 healthy subjects, 6 to 18 years old. Obesity (Silver Spring) 2006;14:819–825. doi: 10.1038/oby.2006.95. [DOI] [PubMed] [Google Scholar]
- 24.Ashby RL, Ward KA, Roberts SA, Edwards L, Mughal MZ, Adams JE. A reference database for the Stratec XCT-2000 peripheral quantitative computed tomography (pQCT) scanner in healthy children and young adults aged 6-19 years. Osteoporos Int. 2008 Dec 6; doi: 10.1007/s00198-008-0800-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Ward KA, Ashby RL, Roberts SA, Adams JE, Zulf Mughal M. UK reference data for the Hologic QDR Discovery dual-energy x ray absorptiometry scanner in healthy children and young adults aged 6-17 years. Arch Dis Child. 2007;92:53–59. doi: 10.1136/adc.2006.097642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duke PM, Litt IF, Gross RT. Adolescents’ self-assessment of sexual maturation. Pediatrics. 1980;66:918–920. [PubMed] [Google Scholar]
- 27.Tanner JM. Growth at adolescence: with a general consideration of the effects of hereditary and environmental factors upon growth and maturation from birth to maturity. Blackwell Scientific Publications; Oxford: 1969. [Google Scholar]
- 28.Wardhaugh B. [Accessed September 2003];Pubertal staging. 2003 Available online: http://www.endocrinology.org/SFE/training/ent00/ent00_war.htm.
- 29.National Osteoporosis Society . A practical guide to bone densitometry in children. Camerton; Bath, UK: 2004. [Google Scholar]
- 30.Thomas SR, Kalkwarf HJ, Buckley DD, Heubi JE. Effective dose of dual-energy X-ray absorptiometry scans in children as a function of age. J Clin Densitom. 2005;8:415–422. doi: 10.1385/jcd:8:4:415. [DOI] [PubMed] [Google Scholar]
- 31.Watson SJ, Jones AL, Oatway WB, Hughes JS. Ionising Radiation Exposure of the UK Population: 2005 review. Health Protection Agency Centre for Radiation, Chemical and Environmental Hazards Radiation Protection Division; Chilton, Didcot, Oxfordshire, UK: 2005. [Google Scholar]
- 32.Landau S, Everitt B. A handbook of statistical analyses using SPSS. Chapman & Hall/CRC; Boca Raton, Florida: 2004. [Google Scholar]
- 33.Lunt M, Felsenberg D, Reeve J, Benevolenskaya L, Cannata J, Dequeker J, Dodenhof C, Falch JA, Masaryk P, Pols HA, Poor G, Reid DM, Scheidt-Nave C, Weber K, Varlow J, Kanis JA, O’Neill TW, Silman AJ. Bone density variation and its effects on risk of vertebral deformity in men and women studied in thirteen European centers: the EVOS Study. J Bone Miner Res. 1997;12:1883–1894. doi: 10.1359/jbmr.1997.12.11.1883. [DOI] [PubMed] [Google Scholar]
- 34.Ismail AA, Pye SR, Cockerill WC, Lunt M, Silman AJ, Reeve J, Banzer D, Benevolenskaya LI, Bhalla A, Bruges Armas J, Cannata JB, Cooper C, Delmas PD, Dequeker J, Dilsen G, Falch JA, Felsch B, Felsenberg D, Finn JD, Gennari C, Hoszowski K, Jajic I, Janott J, Johnell O, Kanis JA, Kragl G, Lopez Vaz A, Lorenc R, Lyritis G, Marchand F, Masaryk P, Matthis C, Miazgowski T, Naves-Diaz M, Pols HA, Poor G, Rapado A, Raspe HH, Reid DM, Reisinger W, Scheidt-Nave C, Stepan J, Todd C, Weber K, Woolf AD, O’Neill TW. Incidence of limb fracture across Europe: results from the European Prospective Osteoporosis Study (EPOS) Osteoporos Int. 2002;13:565–571. doi: 10.1007/s001980200074. [DOI] [PubMed] [Google Scholar]
- 35.Forwood MR, Bailey DA, Beck TJ, Mirwald RL, Baxter-Jones AD, Uusi-Rasi K. Sexual dimorphism of the femoral neck during the adolescent growth spurt: a structural analysis. Bone. 2004;35:973–981. doi: 10.1016/j.bone.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Iuliano-Burns S, Mirwald RL, Bailey DA. Timing and magnitude of peak height velocity and peak tissue velocities for early, average, and late maturing boys and girls. Am J Hum Biol. 2001;13:1–8. doi: 10.1002/1520-6300(200101/02)13:1<1::AID-AJHB1000>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 37.Neu CM, Rauch F, Rittweger J, Manz F, Schoenau E. Influence of puberty on muscle development at the forearm. Am J Physiol Endocrinol Metab. 2002;283:E103–107. doi: 10.1152/ajpendo.00445.2001. [DOI] [PubMed] [Google Scholar]
- 38.Rauch F. Bone growth in length and width: the yin and yang of bone stability. J Musculoskelet Neuronal Interact. 2005;5:194–201. [PubMed] [Google Scholar]
- 39.Rauch F, Schoenau E. The developing bone: slave or master of its cells and molecules? Pediatr Res. 2001;50:309–314. doi: 10.1203/00006450-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Frost HM. The mechanostat: a proposed pathogenic mechanism of osteoporoses and the bone mass effects of mechanical and nonmechanical agents. Bone Miner. 1987;2:73–85. [PubMed] [Google Scholar]
- 41.van der Meulen MC, Ashford MW, Jr., Kiratli BJ, Bachrach LK, Carter DR. Determinants of femoral geometry and structure during adolescent growth. J Orthop Res. 1996;14:22–29. doi: 10.1002/jor.1100140106. [DOI] [PubMed] [Google Scholar]
- 42.Zanchetta JR, Plotkin H, Alvarez Filgueira ML. Bone mass in children: normative values for the 2-20-year-old population. Bone. 1995;16:393S–399S. doi: 10.1016/8756-3282(95)00082-o. [DOI] [PubMed] [Google Scholar]
- 43.van der Sluis IM, de Ridder MA, Boot AM, Krenning EP, de Muinck Keizer-Schrama SM. Reference data for bone density and body composition measured with dual energy x ray absorptiometry in white children and young adults. Arch Dis Child. 2002;87:341–347. doi: 10.1136/adc.87.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schoenau E, Neu CM, Rauch F, Manz F. The development of bone strength at the proximal radius during childhood and adolescence. J Clin Endocrinol Metab. 2001;86:613–618. doi: 10.1210/jcem.86.2.7186. [DOI] [PubMed] [Google Scholar]
- 45.Neu CM, Rauch F, Manz F, Schoenau E. Modeling of cross-sectional bone size, mass and geometry at the proximal radius: a study of normal bone development using peripheral quantitative computed tomography. Osteoporos Int. 2001;12:538–547. doi: 10.1007/s001980170074. [DOI] [PubMed] [Google Scholar]
- 46.Molgaard C, Michaelsen KF. Changes in body composition during growth in healthy school-age children. Appl Radiat Isot. 1998;49:577–579. doi: 10.1016/s0969-8043(97)00076-6. [DOI] [PubMed] [Google Scholar]
- 47.Maynard LM, Guo SS, Chumlea WC, Roche AF, Wisemandle WA, Zeller CM, Towne B, Siervogel RM. Total-body and regional bone mineral content and areal bone mineral density in children aged 8-18 y: the Fels Longitudinal Study. Am J Clin Nutr. 1998;68:1111–1117. doi: 10.1093/ajcn/68.5.1111. [DOI] [PubMed] [Google Scholar]
- 48.Magarey AM, Boulton TJ, Chatterton BE, Schultz C, Nordin BE, Cockington RA. Bone growth from 11 to 17 years: relationship to growth, gender and changes with pubertal status including timing of menarche. Acta Paediatr. 1999;88:139–146. doi: 10.1080/08035259950170286. [DOI] [PubMed] [Google Scholar]
- 49.Landin L, Nilsson BE. Forearm bone mineral content in children. Normative data. Acta Paediatr Scand. 1981;70:919–923. doi: 10.1111/j.1651-2227.1981.tb06251.x. [DOI] [PubMed] [Google Scholar]
- 50.Faulkner RA, Bailey DA, Drinkwater DT, McKay HA, Arnold C, Wilkinson AA. Bone densitometry in Canadian children 8-17 years of age. Calcified Tissue International. 1996;59:344–351. doi: 10.1007/s002239900138. [DOI] [PubMed] [Google Scholar]
- 51.Baxter-Jones AD, Mirwald RL, McKay HA, Bailey DA. A longitudinal analysis of sex differences in bone mineral accrual in healthy 8-19-year-old boys and girls. Ann Hum Biol. 2003;30:160–175. doi: 10.1080/0301446021000034642. [DOI] [PubMed] [Google Scholar]
- 52.van Coeverden SC, Netelenbos JC, de Ridder CM, Roos JC, Popp-Snijders C, Delemarre-van de Waal HA. Bone metabolism markers and bone mass in healthy pubertal boys and girls. Clin Endocrinol (Oxf) 2002;57:107–116. doi: 10.1046/j.1365-2265.2002.01573.x. [DOI] [PubMed] [Google Scholar]
- 53.Wang Q, Alen M, Nicholson PH, Halleen JM, Alatalo SL, Ohlsson C, Suominen H, Cheng S. Differential effects of sex hormones on peri- and endocortical bone surfaces in pubertal girls. J Clin Endocrinol Metab. 2006;91:277–282. doi: 10.1210/jc.2005-1608. [DOI] [PubMed] [Google Scholar]
- 54.Frost HM. On the estrogen-bone relationship and postmenopausal bone loss: A new model. J Bone Miner Res. 1999;14:1473–1477. doi: 10.1359/jbmr.1999.14.9.1473. [DOI] [PubMed] [Google Scholar]
- 55.Libanati C, Baylink DJ, Lois-Wenzel E, Srinvasan N, Mohan S. Studies on the potential mediators of skeletal changes occurring during puberty in girls. J Clin Endocrinol Metab. 1999;84:2807–2814. doi: 10.1210/jcem.84.8.5905. [DOI] [PubMed] [Google Scholar]
- 56.Wang Q, Alen M, Nicholson P, Lyytikainen A, Suuriniemi M, Helkala E, Suominen H, Cheng S. Growth patterns at distal radius and tibial shaft in pubertal girls: a 2-year longitudinal study. J Bone Miner Res. 2005;20:954–961. doi: 10.1359/JBMR.050110. [DOI] [PubMed] [Google Scholar]
- 57.Hogler W, Blimkie CJ, Cowell CT, Kemp AF, Briody J, Wiebe P, Farpour-Lambert N, Duncan CS, Woodhead HJ. A comparison of bone geometry and cortical density at the mid-femur between prepuberty and young adulthood using magnetic resonance imaging. Bone. 2003;33:771–778. doi: 10.1016/s8756-3282(03)00266-7. [DOI] [PubMed] [Google Scholar]
- 58.Bechtold S, Rauch F, Noelle V, Donhauser S, Neu CM, Schoenau E, Schwarz HP. Musculoskeletal analyses of the forearm in young women with Turner syndrome: a study using peripheral quantitative computed tomography. J Clin Endocrinol Metab. 2001;86:5819–5823. doi: 10.1210/jcem.86.12.8063. [DOI] [PubMed] [Google Scholar]
- 59.Parfitt AM. The attainment of peak bone mass: what is the relationship between muscle growth and bone growth? Bone. 2004;34:767–770. doi: 10.1016/j.bone.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 60.Seeman E, Hopper JL, Young NR, Formica C, Goss P, Tsalamandris C. Do genetic factors explain associations between muscle strength, lean mass, and bone density? A twin study. Am J Physiol. 1996;270:E320–327. doi: 10.1152/ajpendo.1996.270.2.E320. [DOI] [PubMed] [Google Scholar]
- 61.Ferretti JL, Cointry GR, Capozza RF. Noninvasive analysis of bone mass, structure and strength. In: An YH, editor. Orthopaedic issues in osteoporosis. CRC Press; Florida: 2002. pp. 145–167. [Google Scholar]