Abstract
The effects of 2-chloroethylphosphonic acid (Ethrel), ethylene, and some growth retardants on sex expression of cucumber plants (Cucumis sativus L.) were investigated, with the use of a monoecious cultivar (Improved Long Green) which has a strong tendency toward maleness.
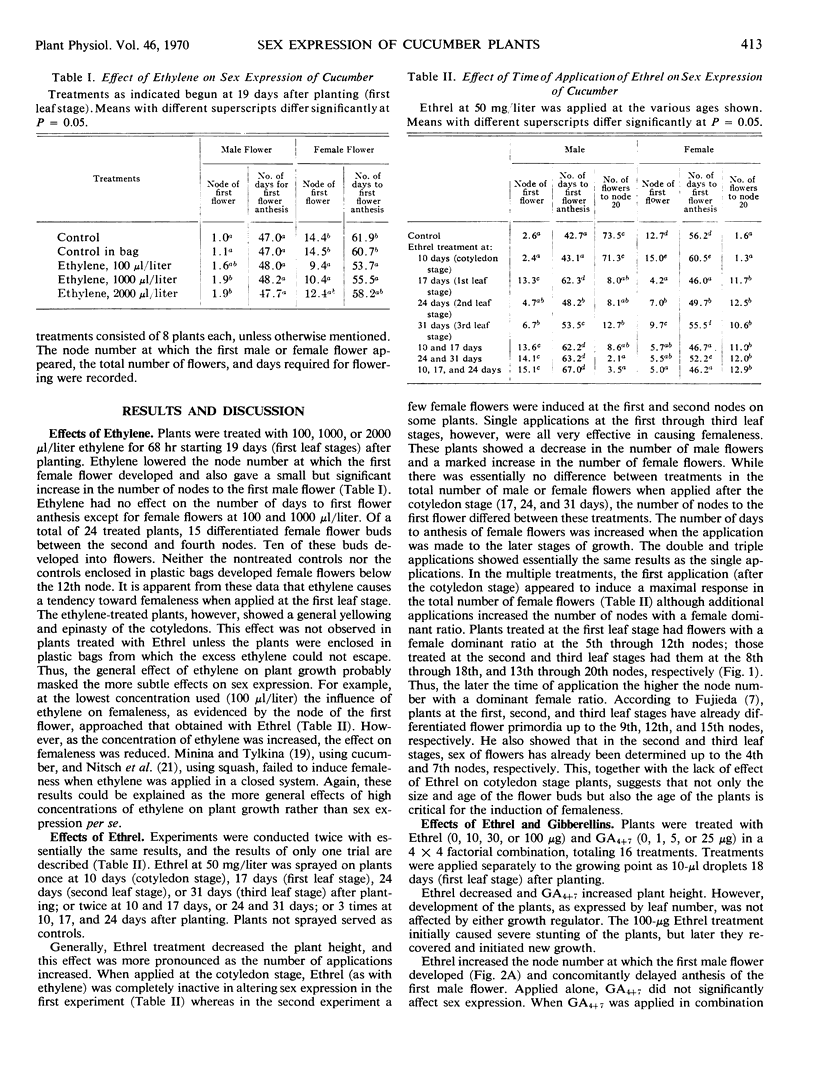
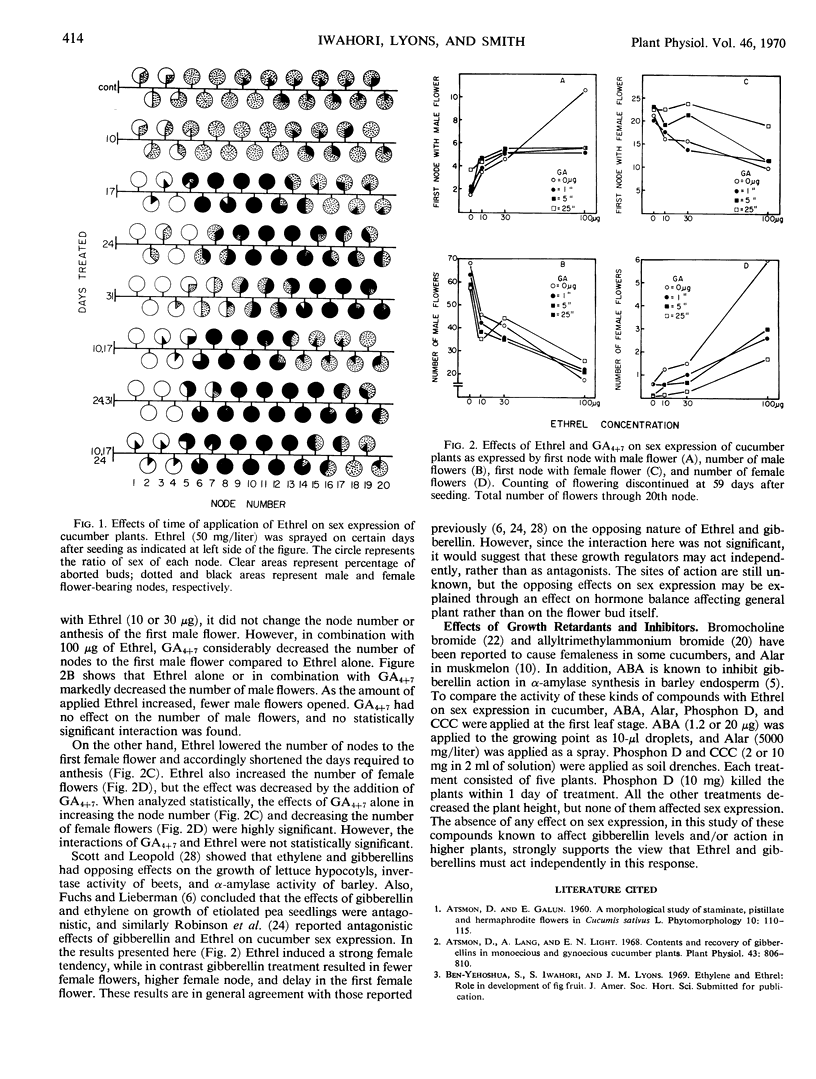
Ethrel caused increased femaleness when applied at 50 milligrams per liter at the first to the third leaf stage, but when applied at the cotyledon stage it was ineffective. The later the time of application, the higher the node at which the first female flower appeared. The total number of female flowers was about the same regardless of application time. A mixture of gibberellins A4 and A7 caused maleness, and Ethrel caused femaleness. However, when applied in combination at the first leaf stage the interaction was not significant. It seems, therefore, that Ethrel and gibberellins are not antagonistic but rather have different sites of action, although they have opposing effects on sex expression.
Ethylene caused femaleness but was far less effective than Ethrel. Alar (N-dimethylaminosuccinamic acid), CCC((2-chloroethyl)trimethylammonium chloride), Phosphon D(2, 4-dichlorobenzyl-tributylphosphonium chloride), and abscisic acid did not affect sex expression of cucumber.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atsmon D., Lang A., Light E. N. Contents and recovery of gibberellins in monoecious and gynoecious cucumber plants. Plant Physiol. 1968 May;43(5):806–810. doi: 10.1104/pp.43.5.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURG S. P., BURG E. A. ETHYLENE ACTION AND THE RIPENING OF FRUITS. Science. 1965 May 28;148(3674):1190–1196. doi: 10.1126/science.148.3674.1190. [DOI] [PubMed] [Google Scholar]
- Fuchs Y., Lieberman M. Effects of Kinetin, IAA, and Gibberellin on Ethylene Production, and Their Interactions in Growth of Seedlings. Plant Physiol. 1968 Dec;43(12):2029–2036. doi: 10.1104/pp.43.12.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galun E., Izhar S., Atsmon D. Determination of Relative Auxin Content in Hermaphrodite and Andromonoecious Cucumis sativus L. Plant Physiol. 1965 Mar;40(2):321–326. doi: 10.1104/pp.40.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson D. E., Wilson P. W., Kodicek E. New vitamin D metabolite localized in intestinal cell nuclei. Nature. 1969 Apr 12;222(5189):171–172. doi: 10.1038/222171a0. [DOI] [PubMed] [Google Scholar]
- McMurray A. L., Miller C. H. Cucumber sex expression modified by 2-chloroethanephosphonic acid. Science. 1968 Dec 20;162(3860):1397–1398. doi: 10.1126/science.162.3860.1397. [DOI] [PubMed] [Google Scholar]
- Mitchell W. D., Wittwer S. H. Chemical Regulation of Flower Sex Expression and Vegetative Growth in Cucumis sativus L. Science. 1962 Jun 8;136(3519):880–881. doi: 10.1126/science.136.3519.880. [DOI] [PubMed] [Google Scholar]
- Peterson C. E., Anhder L. D. Induction of Staminate Flowers on Gynoecious Cucumbers with Gibberellin A3. Science. 1960 Jun 3;131(3414):1673–1674. doi: 10.1126/science.131.3414.1673. [DOI] [PubMed] [Google Scholar]
- Scott P. C., Leopold A. C. Opposing effects of gibberellin and ethylene. Plant Physiol. 1967 Jul;42(7):1021–1022. doi: 10.1104/pp.42.7.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. F. Ethylene evolution from 2-chloroethylphosphonic Acid. Plant Physiol. 1969 Aug;44(8):1203–1204. doi: 10.1104/pp.44.8.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]


