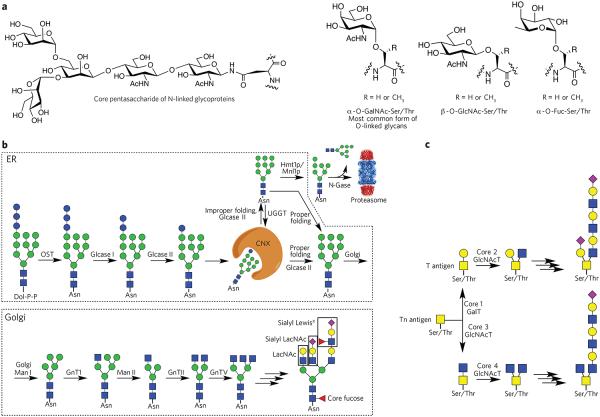
Figure 1. Various classes of glycans and their biosynthesis.
(a) Common types of protein glycosylation: core pentasaccharide common to all N-linked glycans and various types of O-glycosylation. (b) Biosynthesis of N-glycans. The biosynthesis of N-glycans is initiated in the ER by the transfer of a dolichol (Dol-P-P)-linked glycan to an asparagine moiety of an Asn-Xaa-Ser/Thr sequon of a polypeptide. In the ER, protein glycosylation functions in the quality control of protein biosynthesis. Calnexin (CNX) is a chaperone that keeps the unfolded protein in the ER. Improperly folded glycans can encounter the α-mannosidase MNS1, which removes a terminal mannoside, providing a glycoform that will be transported to the cytosol. Here, an N-glycanase (N-Gase) removes the N-linked glycans, and the resulting protein is then imported into the proteasome for proteolysis. Properly folded proteins are transported to the Golgi, where an array of glycosyltransferases diversifies the various antennae of the glycans to give an array of complex structures. The resulting complex-type glycoproteins are transported to the cell surface or secreted. OST, oligosaccharyltransferase; glcase, α-glucosidase; UGGT, UDP–glucose-glycoprotein glucosyltransferase; Hmt1p, HnRNP methyltransferase 1; Mnl1p, mannosidase-like protein 1; N-gase, N-glucosidase; Man, α-mannosidase; GnT, N-acetyl glucosaminyltransferase. (c) Biosynthesis of O-glycans. The biosynthesis of O-glycans that start the structure with an O-GalNAc moiety begins in the Golgi, where various polypeptide polypeptide GalNAc transferases (GlcNAcT) attach a GalNAc residue. The resulting glycoprotein can be converted into various core structures that can be diversified by a range of glycosyltransferases.