Abstract
Osteoimmunology is the crosstalk between the skeletal and immune system. We have previously shown in vitro that osteoclasts (OC) crosspresent antigens to induce FoxP3 in CD8 T-cells (OCiTcREG), which then suppress osteoclast activity. Here we assessed the ability of OC-iTcREG to limit bone resorption in vivo. Mice lacking CD8 T-cells lose more bone in response to RANKL (Tnfsf11) administration. Using adoptive transfer experiments we demonstrate that FoxP3+ CD8 T-cells limit bone loss by RANKL administration. In ovariectomized mice, a murine model of postmenopausal osteoporosis, OC-iTcREG limited bone loss and increased bone density as assessed by serum markers, micro computed tomography (μCT) and histomorphometry. Indeed, OC-iTcREG—treated ovariectomized mice had decreased levels of effector T-cells in the bone marrow compared to untreated mice, and increased bone formation rates relative to bisphosphonate-treated mice. Our results provide the first in vivo evidence that OC-iTcREG have anti-resorptive activity and repress the immune system, thus extending the purview of osteoimmunology.
Keywords: Osteoimmunology, FoxP3 CD8 T-cells, Osteoclasts, Osteoporosis, Negative feedback
Introduction
Bone is remodeled throughout the life of an organism. Bone homeostasis is regulated by a number of regulatory feedback loops that respond to changes occurring all over the body, including mechanical loading (Wolfe’s law). As the bone is a major store for the minerals calcium and phosphate, calcitonin and parathyroid hormone (PTH) regulate the balance between formation and resorption, respectively [1]. Osteoclasts (OC) are the body’s major, if not sole, bone resorbing cells [2] and osteoblasts are the primary bone forming cells. Bone is also a major storage site for growth factors, notably TGFβ and the action of OC on the bone releases the active form of the cytokine [3]. More recently, it has been recognized that cells in the bone not only respond to signals (like PTH), but also release growth factors like osteocalcin that alter the fat and energy metabolism in the body [4].
Bone turnover is also regulated by the immune system. The crosstalk between the bone and immune system has been termed osteoimmunology [5]. Osteoimmunology arose from the recognition that cytokines produced by activated pro-inflammatory effector T-cells (TEFF) promote bone erosion in chronic pathologies like rheumatoid arthritis [6] and osteoporosis [7]. The generation of TEFF is needed to clear pathogens, but their activation must be regulated. Additionally, the random nature by which the B-cell and T-cell repertoire is generated, and because of the limits of central tolerance, there is a constant risk of anti-self responses by cells of the adaptive immune system. Therefore, generation of TEFF to pathogens must be measured and regulated because of the potential damage to the host. One of the negative regulatory mechanisms used by the immune system are regulatory T-cells (TREG). The transcription factor FoxP3 and CD25, the α chain of the IL-2 receptor are markers of TREG. TREG suppress the aberrant activation of self-reactive T-cells [8]. TREG also produce cytokine- and cell-surface mediators that are anti-inflammatory [9]. The balance between effector and regulatory T-cells is central to immune homeostasis: dominant TREG activity may compromise the immune response to pathogens and must be exquisitely regulated. In contrast, overactive effector T-cells (TEFF) may initiate autoimmunity and are kept in check by TREG. While the pro-resorptive effects of TEFF-produced proinflammatory cytokines is well documented in the field of osteoimmunology, the anti-resorptive effects mediated by T-cells has just begun to emerge [10, 11].
We discovered using time course microarray data that differentiating osteoclasts coordinately upregulate expression of genes for a number of pro-angiogenic factors, chemokines and the antigen cross-presentation pathway [12]. We showed that the secreted chemokines recruit CD8 T-cells. We have also demonstrated that mature OC present antigens on MHC class I from exogenous proteins. This activity, called cross-presentation was unexpected as it is typically associated with professional antigen presenting cells. Cross-presentation by OC activated CD8 T-cells. Upon analyzing the phenotype of osteoclast-induced CD8 T-cells we found that they express FoxP3 and CD25[13]. Cross-presentation of antigens by OC uniquely induces a regulatory T-cell phenotype in the CD8 T-cells[13]. In contrast, antigen cross-presentation by dendritic cells to CD8 T-cells results in cytolytic phenotype[13]. As expected, TcREG can suppress the activation and proliferation of CD4 (this work) and CD8 T-cells by dendritic cells in culture[13]. More recently, based on the cytokines produced by TcREG we found that, in vitro, the OC-induced CD8 regulatory T-cells (OC-iTcREG) negatively regulate OC differentiation and resorption activity, to form a negative feedback loop [11]. In the current work we assessed the ability of the OC-iTcREG to negatively regulate bone resorption and the immune system in vivo.
Results
At the same time as the discovery that pro-inflammatory TEFF promote bone erosion, experiments showed that T-cells, CD8 T-cells in particular, were protective against bone loss [14, 15]. For instance, it was noted that when bone marrow cells from TCRα−/− mice, that lack CD4 and CD8 T-cells, were cultured in the presence of 1,25(OH)2 vitamin D3 osteoclastogenesis was enhanced indicating that T-cells suppress osteoclastogenesis[16]. Conversely, while immunocompromised or T-cell deficient mice have not been reported to have overt gross skeletal abnormalities, it has been noted that athymic mice have lower baseline bone mass [17, 18]. As noted above, many regulatory layers control skeletal homeostasis, therefore defects may be subtle and only detected upon perturbation or stressing the system.
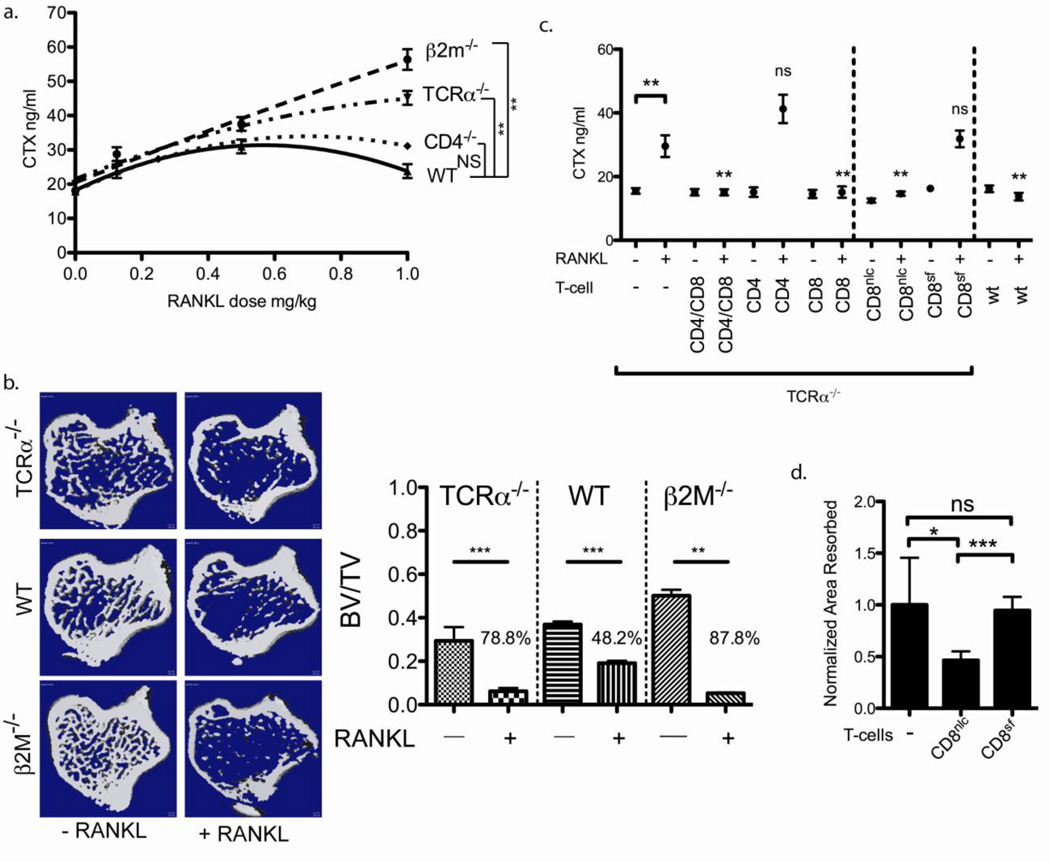
Mice lacking CD8 T-cells have increased bone resorption in response to RANKL
To investigate whether T-cell deficient mice have a defect in maintaining skeletal homeostasis, we perturbed the skeletal system with RANKL because it directly activates osteoclast activity and because RANKL administration has been previously characterized in mice[19]. To determine the role of T-cells, we performed a RANKL dose titration on T-cell sufficient (WT), or mice deficient in CD4 (CD4−/−), CD8 (β2m−/−) or both CD4 and CD8 T-cells (TCRα−/−). Since RANKL activates osteoclasts globally, we initially measured serum carboxyl-terminal collagen telopeptide crosslinks (CTX) to assess the level of overall bone resorption. CD8 T-cell-deficient mice had elevated bone resorption as measured by serum CTX levels at higher (> 0.5 mg/kg) RANKL doses than the CD8 T-cell sufficient mice (Fig. 1A). To confirm the results of the serum CTX we also performed μCT of β2M−/− (deficient in CD8 T-cells), TCRα−/− (deficient in both CD4 and CD8 T-cells) and wildtype (C57BL/6) mice treated with 1 mg/kg RANKL. β2M−/− mice lost more bone in response to 1 mg/kg RANKL relative to TCRα−/− mice. Surprisingly, β2M−/− mice had significantly more bone at baseline (Fig. 1B and Fig. S1). Mice deficient in CD8 T-cell (β2M−/−) or both CD4 and CD8 T-cells (TCRα−/−) lost more bone volume (in the tibia) in response to 1 mg/kg RANKL relative to wildtype mice, confirming the results of serum CTX (Fig. 1B and Fig S1). Our results indicate that mice with CD8 T-cells are able to regulate bone resorption and thus maintain skeletal homeostasis better than CD8 deficient mice. One concern with this experiment is that the different mouse strains may respond differently to RANKL due, for instance to compensatory changes or due to alteration in their microbiomes [20].
Figure 1. FoxP3+ CD8 T-cells limit bone loss.
a) RANKL dose titration into CD8 T-cell sufficient (wt C57BL/6 and CD4−/−) and CD8 T-cell deficient (β2M−/− and TCRα−/−) mice. Mice (n= 4 to 7 mice/group) that had CD8 T-cells have a lower bone resorption relative to those mice that lack CD8 T-cells at dose > 0.5 mg/kg. b) CD8 deficient mice (β2m−/− and TCRα−/−) mice lost more trabecular bone relative to wild-type mice in response to 1 mg/kg RANKL as measured by μCT (n = 6 mice/group). CD8 T-cell deficient β2M−/− mice have significantly increased BV/TV relative to WT and TCRα-−/− (both P = 0.0007) mice. Fig. S1 provides additional analysis of trabecular parameters. A two-way ANOVA was performed using WT and each targeted knockout strain. The analysis indicates that RANKL treatment accounts for 80.0 % of the total variance (P < 0.0001) and strain differences account for 9.5% of the variance (P < 0.0001); the interaction term accounts for the remaining 10.5% (P < 0.0001) of the variance. c) Reconstitution of TCRα−/− mice with CD8 T-cells but not CD4 T-cells limited bone loss in response to 1 mg/kg RANKL. The CD8 T-cells from rescued Scurfy[50] (CD8Sf) mice into TCRα−/− mice did not limit bone loss in response to RANKL, as compared to T-cells from the normal littermate controls (CD8nlc). P values were obtained by comparing CTX values of reconstituted to un-reconstituted TCRα−/− mice. d) In contrast to OC-TcREG generated using purified CD8 T-cells from normal littermate controls, the OC-iTcREG generated from Scurfy mice cannot suppress osteoclast-pitting activity. * = P ≤ 0.05, ** = P ≤ 0.01 and NS = not statistically significant, determined using Mann-Whitney two-sided U-test.
To allay such concerns and to perform a direct comparison of the T-cells in the same strain, we reconstituted TCRα−/− mice with CD8, CD4, or both CD4 and CD8 T-cells. After a 10-day incubation period to allow the transferred cells to achieve steady state levels in the lymphopenic mice (i.e. homeostatic proliferation[21]), the mice were injected with RANKL. Recipients of CD8 T-cell had lower bone resorption than mice receiving CD4 alone (Fig. 1C). These results conclusively show that CD8 T-cells limit bone resorption. To evaluate the need for FoxP3 expression in the CD8 T-cells for limiting bone resorption we used CD8 T-cells from Scurfy mice. Scurfy mice have a frame shift mutation that results in a non-functional (inability to bind DNA) FoxP3 protein that is encoded on the X chromosome [22, 23]. Absence of FoxP3 in hemizygous males is lethal due to multi-organ autoimmune disease by four weeks of age. We rescued these mice from autoimmune disease in two different ways to isolate CD8 T-cells from a non-inflamed environment (see Method for details). We observed that OC-induced CD8 T-cells, generated from purified CD8 T-cells from the rescued Scurfy mice could not suppress OC pitting in vitro (Fig 1D). To test the CD8 T-cells from Scurfy mice for their activity in vivo, we reconstituted TCRα−/− mice with CD8 T-cells from either normal littermates (CD8nlc) or rescued FoxP3 null CD8 T-cells (CD8Sf). CD8 T-cells that could not express functional FoxP3 had higher levels of bone resorption equivalent to un-reconstituted mice (Fig. 1C), indicting that FoxP3+ CD8 T-cells limit bone resorption in response to RANKL administration.
OC-iTcREG suppress bone resorption
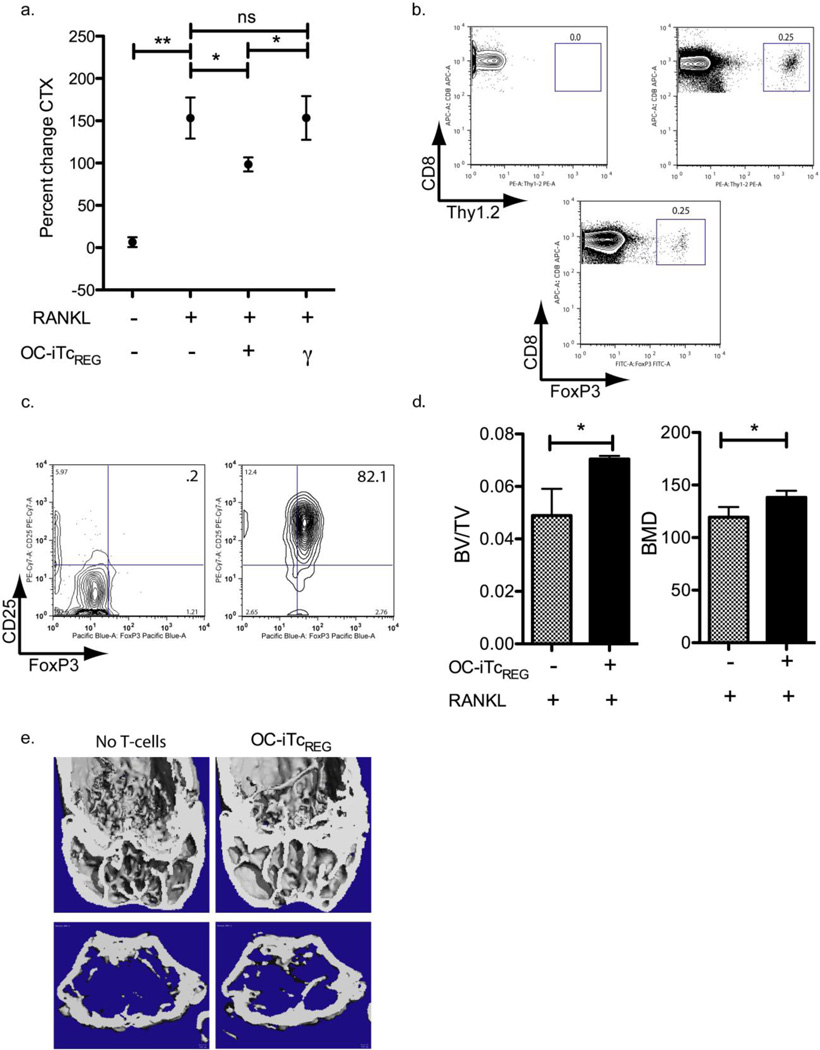
We have previously characterized in detail, the mechanism by which OC-iTcREG suppress osteoclast activity[11]. Furthermore, as osteoclasts induce TcREG which then suppress bone resorption to form a negative feedback loop, we sought to linearize the loop by using ex vivo generated OC-iTcREG. Therefore, based on the results of Fig. 1 we activated osteoclasts using 1 mg/kg dose of RANKL in the presence or absence of preformed OC-iTcREG to test their ability to repress bone resorption in vivo. OC-iTcREG (donors) were generated from ovalbumin-specific OT-I transgenic T-cells and adoptively transferred into recipient OT-I Rag1−/− mouse. The OT-I CD8 T-cells were chosen as donors to test the need for TcREG restimulation (binding of the TCR to it’s cognate antigen) to suppress OC activity. All Tcells produced in OT-I Rag−/− mice express the transgenic T-cell receptor (TCR) that recognizes an ovalbumin peptide (OVA259-263: SIINFEKL) in the context of MHC class I H-2KB. Since mice do not express ovalbumin they cannot present this peptide and restimulate the OC-iTcREG. The OT-I mice were also chosen as recipients because transferring TREG or TcREG into a lymphopenic mouse leads to loss of FoxP3 expression[24] and because they lack endogenous TcREG, and because OT-I mice are not lymphopenic, thus eliminating two potentially confounding experimental conditions. RANKL (1 mg/kg) was injected intraperitoneally twice, 24 hours apart to assess if the OC-iTcREG suppress bone turnover. The mice were sacrificed at 50 h. Bone suppression was assayed by measuring serum CTX. As a control, we used OT-I (CD8) T-cells nonspecifically activated by anti-CD3 and anti-CD28 antibody; T-cells activated in this manner produce significant levels of interferon (IFN)-γ, a known inhibitor of osteoclastogenesis. In contrast to OC-iTcREG—treated mice which showed limited bone turnover, untreated and control IFN-γ producing CD8 T-cell treated mice showed a robust increase in bone resorption in response to RANKL (Fig. 2A). Our results indicate, that unlike CD4 TREG which need to be restimulated through their TCR to mediate suppression[11], TcREG once activated do not require restimulation. In these experiment, the number of adoptively transferred OC-iTcREG was found to be in the physiologically range. As shown in Fig. 2B, the fraction of transferred TcREG (top right) in the bone marrow space was comparable to levels found in a FoxP3eGFP reporter mouse.
Figure 2. Osteoclast-induced TcREG suppress bone resorption in response to RANKL.
a) OC-iTcREG generated from ovalbumin-specific OT-I T-cells could suppress bone turnover in response to RANKL administration (twice at 1 mg/kg, 24 hours apart) as assessed by serum collagen cross-linked telopeptide (CTX) in OT-I mice (n=8 mice/group). b) The levels of OCiTcREG found in the bone marrow after adoptive transfer (top right) are similar to levels found in wildtype mice (bottom). c) Polyclonal TcREG were generated by co-culturing osteoclasts with wild-type splenic CD8 T-cells in the absence (left panel) or presence (right panel) of anti-CD3 antibody. Forty-eight hours after culturing, the cells were stained with flour-conjugated antibodies directed against FoxP3 and CD25 antibody. d) OC-iTcREG generated from polyclonal T-cells using anti-CD3 plus osteoclasts could also suppress bone loss as assessed by μCT of distal femora. The bones (n = 8 mice/group) were harvested 50 h after first dose of RANKL, fixed overnight in 70% ethanol and the tibias subject to μCT. e) Representative images from μCT of distal femora quantitated in panel D are shown.
To evaluate whether the observed effect was unique to the transgenic OT-I OC-iTcREG, we co-cultured polyclonal CD8 T-cells with mature osteoclasts in the presence of anti-CD3 to ligate the TCR of the T-cells. Co-culturing CD8 T-cells under these conditions induced FoxP3 and CD25 (Fig. 2C). These polyclonal OC-iTcREG were then adoptively transferred into OT-I Rag1−/− recipients. After RANKL administration, OC-iTcREG treated mice retained 40% more bone mass (BV/TV) and had higher bone mineral density than untreated controls (Fig. 2D, E and S2). These results indicate that OC-iTcREG protect against bone loss.
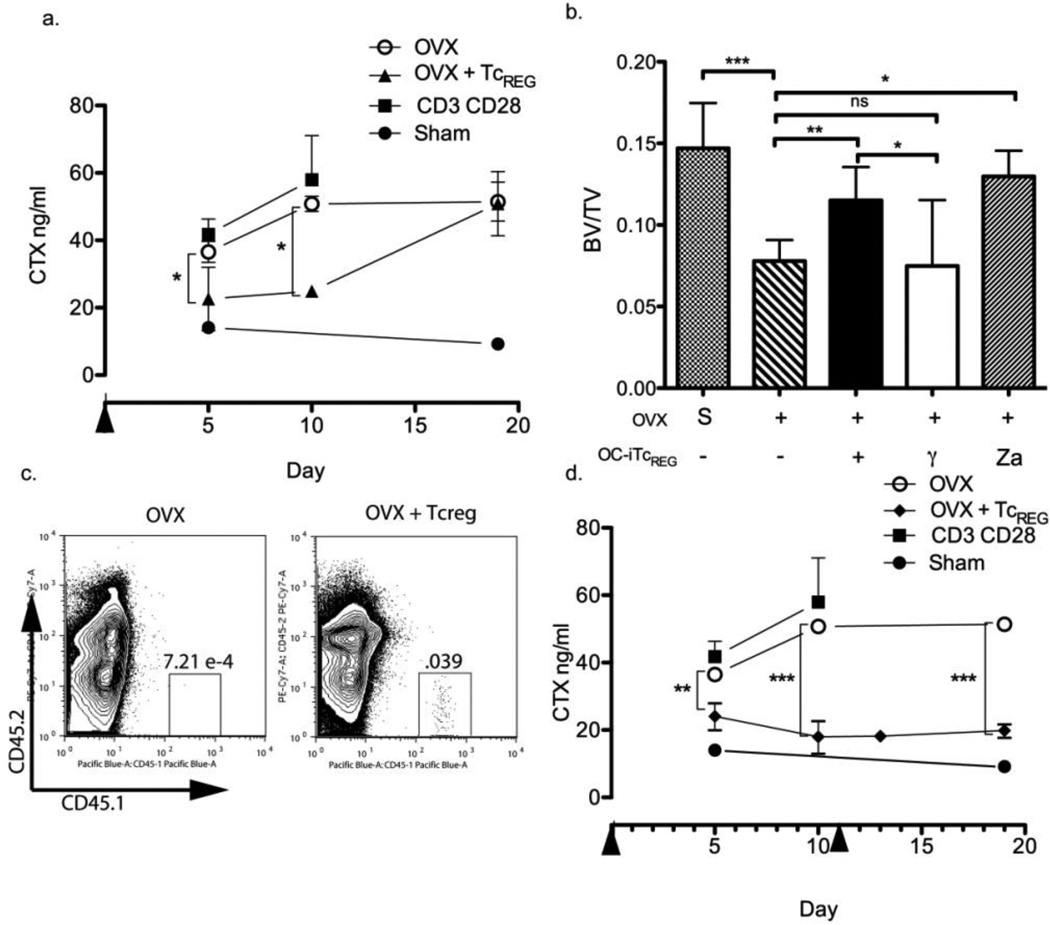
Polyclonal OC-iTcREG limit bone loss in ovariectomized mice
We next investigated whether the OC-iTcREG could reduce bone turnover under a pathological condition. To this end, we used bilaterally ovariectomized (OVX) mice. OVX mimics post-menopausal osteoporosis in mice, a common human disease, by decreasing estrogen levels. We then transferred OC-iTcREG into mice two weeks post-OVX (see methods for details). We waited two weeks post-OVX because increased bone turnover is observed at this time point [25, 26]. Bone resorption was evaluated by serum-CTX ELISA every 5 days. While, untreated OVX mice or those treated with control nonspecifically activated CD8 T-cells showed an increase in bone resorption from day 5 to day 10 post T-cell transfer, mice treated with OC-iTcREG showed no change in bone resorption during the same interval (Fig. 3A). At 19 days post transfer, however, CTX in the OC-iTcREG treated mice had increased to control levels (Fig. 3A) and the OC-iTREG were not detected in the bone marrow (data not shown). We repeated the experiment and harvested tibias for μCT analysis 10 days post transfer to evaluate bone loss. In these latter experiments, we included as a control, the bisphosphonate Zoledronate, which also serves as a comparator for the extent of suppression of bone loss. Our results show that at day 10 post transfer, OC-iTcREG treated mice had an increased bone volume (BV/TV) relative to controls and to a similar extent as Zoledronate (Fig. 3B and S3). Furthermore, the OC-iTcREG were readily detected in the bone marrow of recipient animals (Fig. 3C). Consistent with this result, other experiments indicate that adoptively transferred OC-iTcREG have a half-life of 5 days. To confirm, first that the increase in bone resorption at day 19 was due to decay of the TcREG, and second, if we could maintain decreased bone resorption, we administered a second round of OC-iTcREG. Indeed, with a second treatment, mice showed a sustained decrease in levels of bone resorption (Fig. 3D). These results confirm that OC-iTREG limit bone resorption in ovariectomized mice.
Figure 3. Osteoclast-induced TcREG can limit bone resorption in ovariectomized mice.
a) A single infusion of OC-iTcREG into ovariectomized mice (day 0 indicated by arrowhead is 14 days post-OVX; n = 7 to 8 mice/group) could limit bone resorption (serum CTX) for 10 days. As a control, activated CD8 T-cells that secrete IFN-γ were adoptively transferred (γ). Control T-cells were activated using plate-bound anti-CD3+anti-CD28 antibodies. b) The experiment shown in figure 3a was repeated with mice receiving a single treatment of either no TcREG, OCiTcREG, control non-specifically activated CD8 T-cells, or Zoledronate as a comparator (n= 8 to 9 mice/group). Ovariectomized mice treated with OC-iTcREG had higher BV/TV, comparable to levels achieved by Zoledronate (Za), as assayed by µCT of proximal tibia after 10 days. c) Polyclonal TcREG were generated from CD45.1 marked mice and adoptively transferred into 14-week-old CD45.2 ovariectomized mice. Bone marrow cells from untreated (left; shown for assessment of background) and TcREG-treated mice were harvested on day 10 post-transfer. d) Ovariectomized mice were infused with OC-iTcREG twice (indicated by arrowheads), at day 0 (14 days post-OVX) and day 11 to test the ability of TcREG to sustain decreased bone resorption. * = P ≤ 0.05, ** = P ≤ 0.01, *** = P ≤ 0.001 and NS = not statistically significant determined using Mann-Whitney two-sided U-test.
In the next set of experiments we examined the numbers of osteoclasts, levels of activated effector T-cells, and the bone formation in mice treated with OC-iTcREG to determine to mechanism by which the OC-iTcREG limit bone loss.
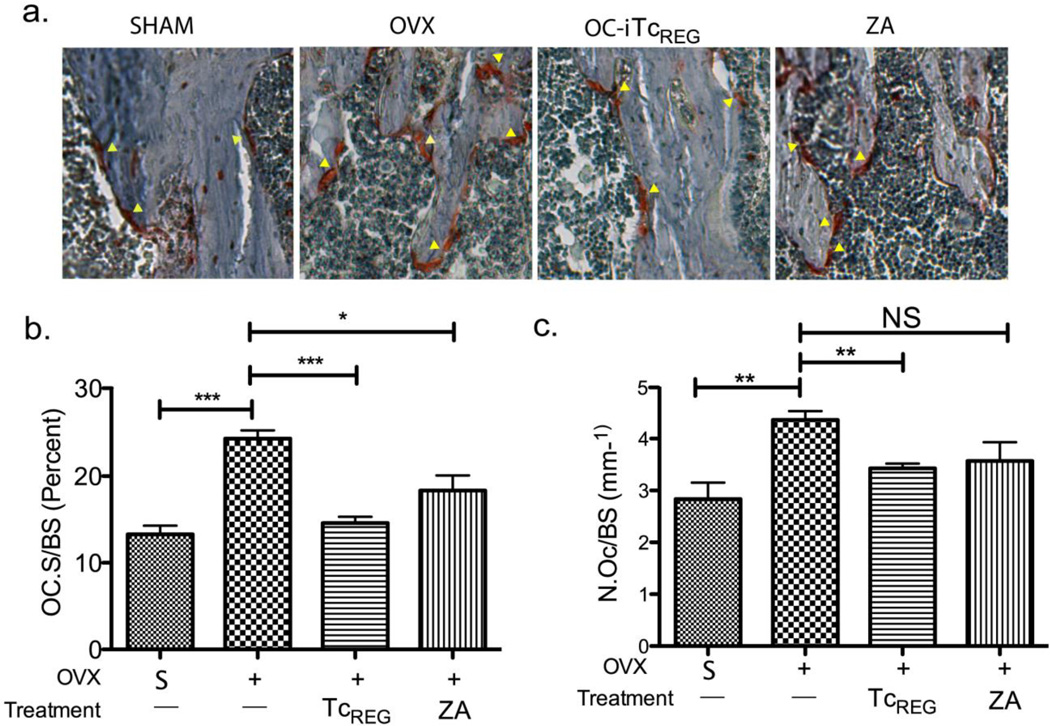
OC-iTcREG-treated ovariectomized mice have fewer osteoclasts
Estrogen deficiency leads to an increase in the numbers of osteoclasts via a Fas ligand-dependent pathway[27]. We have previously shown in vitro that OC-iTcREG inhibit osteoclastogenesis[11]. Therefore, we performed bone histomorphometry to test if OC-iTcREG mice also decrease numbers of osteoclasts in vivo in OC-iTcREG treated mice relative to the untreated controls. Consistent with our previous in vitro studies we find fewer osteoclasts (TRAP positive cells) in OC-iTcREG-treated mice relative to untreated mice and to Zoledronate treated mice (Fig. 4A and B). The percent of bone surface occupied by OC (OC.S/BS) in the tibia (Fig. 4C) also decreased in OC-iTcREG treated mice. These results indicate that OC-iTcREG reduce osteoclast numbers in vivo.
Figure 4. Osteoclast-induced TcREG decrease number of osteoclasts.
a) Ovariectomy increased the number of OC consistent with previous studies[27, 52]. OC-iTcREG treated mice had decreased number of osteoclasts as measured by TRAP staining. The decrease in osteoclasts (in reddish-brown indicated by arrowheads) was confirmed by bone histomorphometry. A representative TRAP staining from each group is shown in panel a. Quantitation of osteoclast surface on bone in tibia in response to TcREG treatment is shown in panel b. A decrease in numbers of osteoclast (panel c) on bone was observed in tibia of mice treated with OC-iTcREG. * = P ≤ 0.05, ** = P ≤ 0.01, *** = P ≤ 0.001 and NS = not statistically significant determined using Mann-Whitney two-sided U-test.
OC-iTcREG-treated ovariectomized mice have decreased levels of effector T-cells
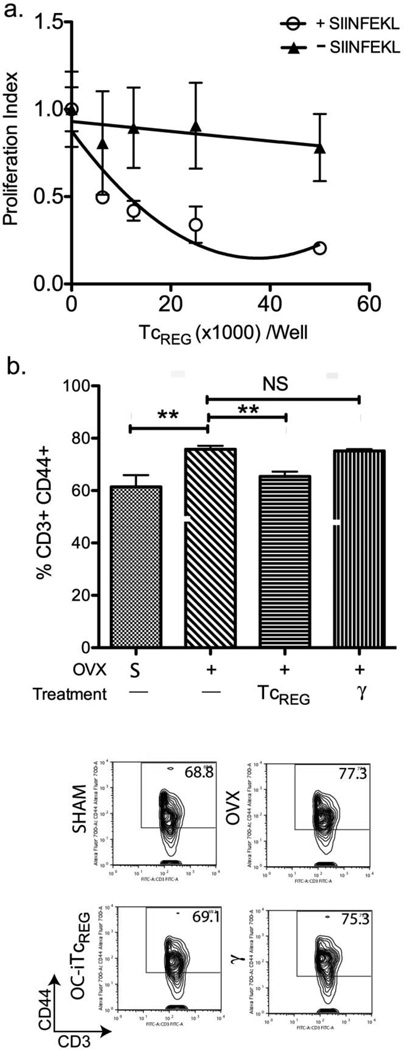
Although controversial [29], it has been suggested proinflammatory cytokines produced by effector T-cells (TEFF) contribute to bone loss in estrogen-depleted animals[7, 25, 30]. Since OC-iTcREG produce the immuno-suppressors IL-10 and CTLA-4, and since we have previously shown that they suppress the activation of naïve CD8 T-cells[13], here we assessed suppression of CD4 T-cells activation by dendritic cells. Responder OT-II CD4 T-cells were labeled with the dye CFSE (to measure proliferation) and co-cultured with purified splenic dendritic cells (DC) in the presence of graded number of OC-iTcREG (details in Methods). To activate the OT-II responder cells, all DC were pulsed with OT-II peptide. To test if suppression by the OC-iTcREG is antigen-specific, in some wells the DC were also pulsed with OT-I peptide. The results show that OC-iTcREG do indeed suppress the proliferation of CD4 T-cells in a dose dependent and antigen-dependent manner because OT-I OC-iTcREG could only suppress in the presence of OT-I peptide (SIINFEKL) (Fig. 5A). Based on these results, we anticipated that since we are adoptively transferring polyclonal T-cells, there should be decreased numbers of activated (CD44+) T-cells (CD3+) in treated relative to untreated mice. Therefore, we measured the relative levels of CD44+ (“antigen experienced”) T-cells in the bone marrow space of sham, OVX and TcREG-treated OVX mice. Our results (Fig. 5B) show that OVX increased levels of TEFF in the bone marrow relative to sham surgery. Indeed, OC-iTcREG treated OVX mice had lowered TEFF levels, as expected (Fig. 5B), indicating that OC-iTcREG have regulatory T-cell activity in vivo.
Figure 5. Treatment of ovariectomized mice with OC-induced TcREG decreased effector T-cells.
A) OC-iTcREG suppress proliferation of CD4 T-cells in an antigen dependent manner. The indicated numbers (50K, 25K, 12.5K, or 6.25K) of osteoclast-induced ovalbumin-specific OT-I TcREG were added with 50,000 CFSE-labeled naïve OT-II CD4 responder T-cells to 50,000 dendritic cells that had been pulsed with ovalbumin peptide (OVA323-339). In addition, some dendritic cells were also pulsed with either OVA257-264 (+ SIINFEKL) or no additional peptide (–SIINFEKL). The amount of proliferation (CFSE-dilution) of responder T-cells was measured by flow-cytometry after 72 hours. B) Ovariectomized mice had increased fraction of effector T-cells (CD3+ and CD44+) relative to sham-operated mice. Treatment of ovariectomized mice with OC-iTcREG decreased the fraction of effector T-cells. No change in the fraction of effector T-cells was observed in ovariectomized mice treated with nonspecifically activated CD8 T-cells (γ). Cells were obtained from femora of mice in Fig. 3B. Representatives FACS plots from each group (as indicated) are shown in the panels below. * = P ≤ 0.05, ** = P ≤ 0.01, *** = P ≤ 0.001 and NS = not statistically significant determined using Mann-Whitney two-sided U-test.
OC-iTcREG treatment of ovariectomized mice increases bone formation rate
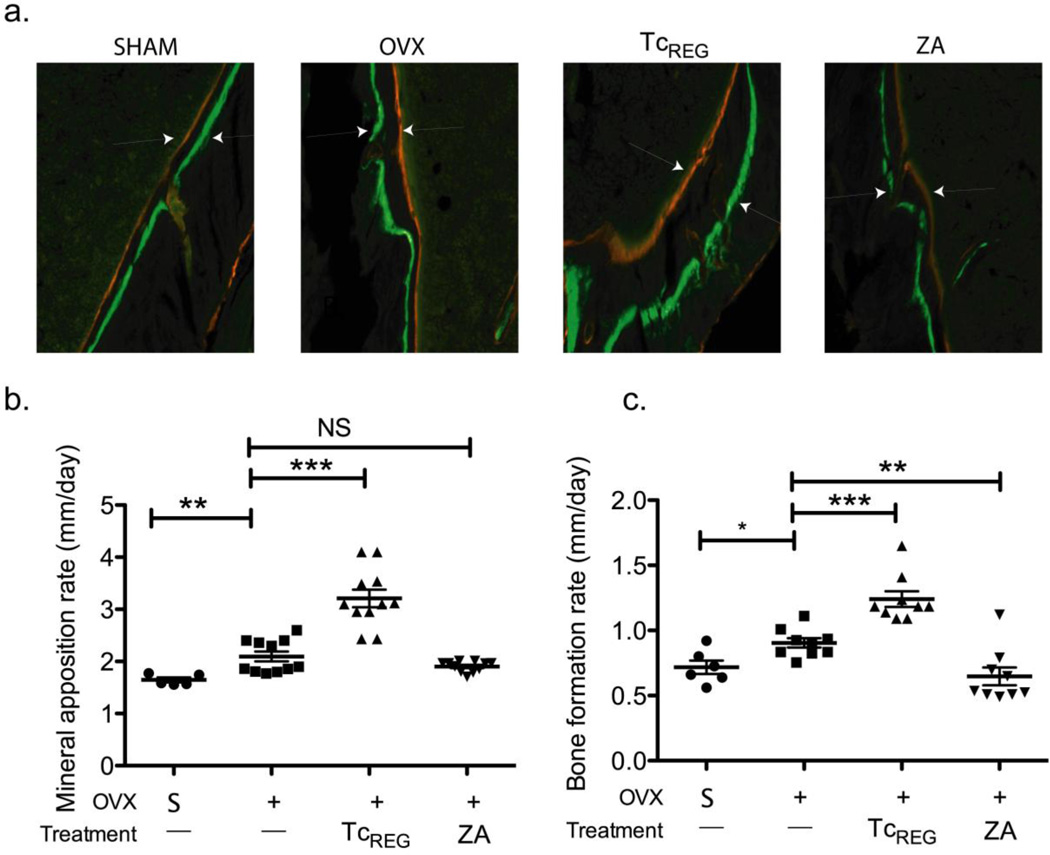
Serum CTX measurements indicate that OC-iTcREG limited bone resorption in OVX mice. Bone parameter measurements by μCT indicate that OC-iTcREG treated OVX mice had higher bone mass and density than untreated mice. To assess the effect of TcREG treatment on bone formation, we injected two dyes that are incorporated into the bone, 10 days apart. The distance between the dyes and the surface area labeled provides the mineral apposition and bone formation rate, respectively. As a control, mice were treated with the bisphosphonate Zoledronate (ZA) to block OC activity[31]. Our results show that OC-iTcREG treatment increased mineral apposition and bone formation rates in OVX mice (Fig. 6). This effect was not observed in ZA treated OVX mice.
Figure 6. Osteoclast-induced TcREG treated mice have higher bone formation rate relative to bisphosphonate-treated mice.
An increase in mineral apposition rate (MAR; panel b) and bone formation rate (BFR; panel c) is observed in OC-iTcREG treated mice relative to untreated mice and to Zoledronate (ZA) treated mice (n = 6 to 11 mice/group). Representative image of double labeled (calcein green and alizarin red) from each group (as indicated) is shown above in panel a. Arrow heads are shown to accent distance between dyes. * P ≤ 0.05, ** = P ≤ 0.01, *** = P ≤ 0.001 and NS = not statistically significant, determined using Mann-Whitney two-sided U-test.
Discussion
We have previously shown that osteoclast-induced TcREG can suppress osteoclast activity in pitting assays performed in culture, to form a negative feedback loop[11]. To assess the ability of T-cells to limit bone turnover, a RANKL dose titration was performed in wildtype mice, and strains that lacked CD4, CD8 T-cells or both CD4 and CD8. Mice deficient in CD8 T-cells had a much higher bone resorption when treated with RANKL at doses > 0.5 mg/kg (Fig. 1A). Reconstitution of mice lacking αβ T-cells with CD8 T-cells that could express FoxP3 was sufficient to limit bone loss and resorption in response to 1 mg/kg RANKL (Fig. 1B and C). The results also demonstrate that CD4 helper functions are not required as TcREG function was observed in CD4−/− and TCRα−/− mice reconstituted with only CD8 T-cells. The results also show that CD4 TREG cannot compensate for loss of TcREG as β2m−/− and TCRα−/− mice reconstituted with complete CD4 T-cells were unable to limit bone resorption. To test for the requirement for functional FoxP3 expression, TCRα−/− mice were reconstituted with CD8 T-cells from Scurfy mice that have genetic lesion in the FoxP3 gene. In contrast to CD8 T-cells from normal littermate controls, CD8 T-cells from Scurfy mice did not limit bone resorption (Fig 1C) in accord with the in vitro matrix dissolution assay (Fig 1D). Our results demonstrate that in vivo the FoxP3+ CD8 T-cells are responsible for the protective bone resorption activity previously described[14–16].
Demonstrating a cause-and-effect relationship for a loop presents a challenge; therefore we sought to linearize the loop: to dissociate the activation and induction of regulatory CD8 T-cells by osteoclasts from the physiological activity of the OC-iTcREG in vivo. In the current study we have demonstrated that ex vivo generated osteoclast-induced TcREG can suppress bone resorption in vivo using two different models. We have previously shown in vitro that OC-iTcREG can block osteoclast precursors from differentiating and cytoskeletal reorganization in mature osteoclasts (see figures 3A and 4 in reference [11]). Here, we attribute suppression of bone resorption in the short-term RANKL administration 50-hour assay, in large part, on suppression of mature osteoclasts by OC-iTcREG. In contrast, in OVX experiments we measured the effect of OC-iTcREG over 10 days. In these longer-term experiments we observe fewer osteoclasts by histomorphometry (Fig. 4), which we attribute to the ability of OC-iTcREG to suppress differentiation. Furthermore, our results indicate that the OC-iTcREG not only limit bone turnover, but also decreased the number of TEFF in ovariectomized mice (Fig. 5) consistent with the regulatory T-cell phenotype observed with TcREG in the in vitro assay[13]. Unexpectedly, the mineral apposition rate (MAR) and bone formation rate (BFR) were increased in the TcREG treated group compared to both the untreated and Zoledronate treated mice (Fig. 6). Since bone resorption and formation are linked, the anabolic and catabolic rates are balanced to maintain bone homeostasis[2]. The decrease in estrogen at menopause increases osteoclast numbers and hence the catabolic rate which tips the balance towards net bone loss. One explanation for this observation of increased bone formation rate is that increasing the pool of OC-iTcREG by adoptive transfer slows down osteoclast activity and allows the osteoblasts to catch up and fill in the previously excavated bone. Unlike Zoledronate, which irreversibly inhibits resorption, OCiTcREG must allow for low-level osteoclast activity and therefore tip the anabolic-catabolic balance back towards homeostasis. The increase in MAR and BFR suggests that OVX not only increases resorption, but also leads to a deficit in bone formation as well. Therefore, another interpretation, that we favor, is that TEFF-produced cytokines repress bone formation. Reduction of TEFF by OC-iTcREG could concurrently reduce bone resorption and derepress bone formation [32, 33]. Zoledronate does not affect the TEFF numbers and therefore does not alter bone formation rate. Additional studies are needed to understand this intriguing observation.
Adoptive transfer of ex vivo generated TREG is being used in clinical trials as immunotherapy, for example in preventing graft vs. host disease for transplantation patients[34]. Adoptive transfer of ex vivo generated OC-iTcREG to treat osteoporosis in a mouse model parallels the immunotherapy experimental design. However, our primary goal was to understand the physiological function of TcREG in the context of osteoimmunology. Compared to the CD4+ TREG which are present at 5 to 12% of CD4 T-cells, TcREG have not been studied in great detail, in part due to their low abundance (0.2 to 1% of CD8 T-cells) in lymphoid tissue. The two regulatory T-cells are controlled differently: thymically and peripherally produced TREG require restimulation through their T-cell receptor (TCR) by MHC class II to express their suppressive effector functions[35]. The maturation of antigen presenting cells that express MHC class II needed for restimulation is stringently regulated[36]. In contrast, TcREG do not require restimulation[11]. In any case, as all cells (except RBC) constitutively express MHC class I, any cell could potentially stimulate TcREG. Our previous studies[11] and others[37–39] have shown that TcREG are regulated by induction locally (e.g. in the bone marrow) from naïve CD8 T-cells; hence their steady state abundance would be low in lymphoid tissue. Numerically, 0.25% (Fig. 2) of CD8 T-cells (~ 4%) in the mouse femur (~ 25 million cells) represents roughly 2,500 OC-iTcREG per femur. Bone remodeling is carried out in spatially discrete foci by a set of cells that form a basic multicellular unit (BMU) or a bone-remodeling compartment (BRC) for cancellous bone. The total numbers of active BMU in the mouse bone marrow is difficult to estimate because of the dynamic nature of the BMUs[40, 41]. Nonetheless, based on in vitro titration data we estimate 2,500 TcREG in the bone marrow space could regulate ~ 500 to 750 actively remodeling foci, given that OC-iTcREG regulate osteoclast activity by diffusible cytokines[11] whose local concentration would be high[13]. Furthermore, the low abundance of a regulator does not belie its importance. Indeed, most regulators are present in low abundance. For instance, transcription factors are present at < 0.1% of cellular proteins[42] are critical regulators of gene expression [43].
Together, our studies establish a novel and unique physiological function for OC-iTcREG: that the negative feedback loop buffers against large changes in bone resorption. For the purposes of this work, it is noteworthy that bone marrow is also the primary site of hematopoiesis and early differentiation of immune cells postnatally in mammals. Large changes in osteoclast activity may be regulated by the immune system to buffer the egress of the hematopoetic precursor cells from the bone marrow[44], which then mature to form cells of the immune system. OC remove bone by secreting acid and proteases into sealed compartments (lacunae) between the OC and the bone. The protein and mineral products of the excavated bone are transcytosed from the lacunae and released through the secretory domain at the apical surface of the OC [45]. Proteomics of the bone matrix shows that nearly 90% of the protein is type I collagen; the remaining 10% consists of type II collagen and over twenty other proteins[46]. Administering collagen (with adjuvant) initiates arthritis (CiA)[47] by activating T-cells[48], indicating that anti-collagen T-cells exist in the normal repertoire of rodents (and humans[49]). On the basis of these observations we suggest that a second physiological role of cross-presentation by OC is to convert autoreactive T-cells into regulatory T-cells so as to prevent autoimmunity in response to neo-antigens released by action of OC on the bone. Importantly, OC-iTcREG are not only anti-resorptive but we also demonstrate that TcREG modulate the immune system by decreasing TEFF number in OVX mice. Our results extend the purview of osteoimmunology by demonstrating bidirectional regulation: OC induce a novel regulatory CD8 T-cell that negatively regulates both the skeletal and the immune systems.
Methods and Materials
Mice
Five-week-old male C57BL/6 mice were purchased from Jackson Labs or used from in-house breeding colonies. Breeders of FoxP3eGFP reporter (model 006769), β2M−/− (model 002807), CD4−/− (model 00263), TCRα−/− (model 00216) mice on a C57BL/6 background were purchased from Jackson Labs, and bred in-house for these experiments. OT-I/Rag−/− mice were purchased from Taconic. All animals were maintained in the Department of Comparative Medicine, Saint Louis University School of Medicine in accordance with institutional and Public Health Service Guidelines. Saint Louis University School of Medicine Institutional Animal Care and Use Committee approved all procedures performed on mice (protocol numbers 2072 and 2184).
Ovariectomy
Bilateral ovariectomy was performed on 12-14 week old mice. Mice were anesthetized using 2.5% isoflurane to initiate anesthesia, and 1% for maintenance. The ovaries were accessed through a single incision in the skin, and exteriorized through muscle wall on each side. Each ovary was clamped using hemostat and removed by a single cut. Skin staples (3M) were used to close the skin incision. To minimize discomfort post-surgery, 0.025 mg/kg Buprenorphine was administered subcutaneously. Zoledronate (Selleck Chemicals) was administered at 30 μg/kg via tail vein.
Adoptive transfer of T-cells
All T-cells were transferred via tail vein. For injections mice were restrained and T-cells, suspended in 100-150 μl PBS were injected into the lateral vein.
Rescue of Scurfy mice
Heterozygote females were purchased from Jackson Laboratory (model 04088) and crossed to a wild-type male. Male pups hemizygous for mutated FoxP3 develop inflammation and multifocal autoimmune disease and die by 25 days of age. To avoid transferring CD8 T-cells from an inflammatory environment and to obtain sufficient cells two approaches were used. In the first approach, all male pups were injected with GFP+ CD4 T-cells (sorted from FoxP3eGFP reporter mice) intraperitoneally at 3 and 20 days of age [50]. After genotyping, the CD8 T-cells were purified (by cell sorting on CD3+, CD4- and CD8+ cells) from the Scurfy positive mice. In the second approach we generated bone marrow chimeras. Bone marrow cells from the 5 to 6 day old (CD45.2+) Scurfy positive mice were mixed with bone marrow from congenically marked CD45.1 mice (at a ratio 5:1 to 8:1 Scurfy to CD45.1). The cells were transferred via tail-vein into ten-week-old sub-lethally irradiated (700 cGy) TCRα−/− mice (3×106 cells/recipient). The CD45.2+ CD8 T-cells were purified by cell sorting 8 to 10 weeks post transfer form spleen and bone marrow.
Generation of OC
OC precursors were isolated as previously described [11, 13]. Briefly, the mice were sacrificed by CO2 asphyxiation and the long bones harvested. The caps of the bones were removed and bone marrow cells were flushed with 0.05% collagenase (Worthington) in α-minimum essential medium (αMEM, Invitrogen). The cell population was filtered through a 40 μ cell strainer, pelleted, resuspended and maintained in αMEM growth medium (αMEM supplemented with 10% heat-inactivated fetal bovine serum [Invitrogen]), penicillin-streptomycin-glutamine (Invitrogen) and recombinant murine M-CSF (Peprotech) at 20 ng/ml). OC were generated by addition of recombinant murine GST-RANKL (a gift of Prof. Steven Teitelbaum, Washington University in St. Louis) to a final concentration of 50 ng/ml. M-CSF and GST-RANKL were added every 48 to 72 h.
Isolation of T-cells
Single cell suspensions of spleens were prepared in PBS + 1% FBS by grinding with a sterile syringe plunger and dispersed by pipetting, then filtering through a 40 μ cell strainer. For co-culture experiments, OT-II CD4 or OT-I CD8 T-cells were prepared by first enriching for T-cells using Pan-T-cell beads then purified by negative selection using appropriate magnetic beads (Miltenyi). All bone marrow and splenic polyclonal T-cells were purified by positive selection and incubated for 30 m at 37° C to allow cells to allow dissociation or uptake of bound beads from cell surface. The resulting T-cells were routinely > 97% pure when stained with anti-CD3, anti-CD4 and anti-CD8 antibody.
Generation of OC-iTcREG
Day 4 OC cultured in 20 ng/ml M-CSF and 50 ng/ml GST-RANKL, were seeded at 5×105 cells/ml/well in the presence of 5 μM OVA (A-5503; Sigma-Aldrich) in 24-well tissue culture-treated plates (Corning). After 14–16 h of incubation, medium was removed and (adherent) cells were washed with pre-warmed medium. 2.5×105 freshly harvested splenic OT-I transgenic T cells purified by negative selection were added in 2 ml of complete T-cell media (RPMI, 10% ΔFBS, penicillin-streptomycin-glutamine, non-essential amino acids, sodium pyruvate, HEPES, and 55 μM β-mercaptoethanol). Following 48 h co-culture, T-cell aliquots were removed and stained intracellularly to assess FoxP3 expression. The TcREG were then further expanded, in the absence of OC, by splitting cells 1:2 and culturing in 100 U/ml IL-2 containing T-cell media for an additional 48 h. For polyclonal TcREG generation, T-cells were purified from spleens of C57BL/6 mice and incubated with day 4 OC in the presence of 1μg/ml anti-CD3 (in lieu of ovalbumin). Control T-cells were activated with plate bound anti-CD3 (1 μg/ml) and anti-CD28 (2μg/ml; both from eBiosciences) for 48 hours; the activated T-cells were expanded further by splitting 1:2 and culturing for additional 48 hours in IL-2 (100 U/ml). 20×106 TcREG (in 200 μL) were then injected by tail vein into 8-week-old OT-I mice.
Antibodies and Fluorescence activated cells sorting (FACS)
Anti-mouse antibodies for FACS were: PE-conjugated anti-mouse CD8a (clone 53-6.7; BD Pharmingen), AF700-conjugated anti-mouse CD44 (IM7; BD Pharmingen), e450-conjugated anti-mouse FoxP3 (FJK-16s, eBioscience), anti-CD3e (500A2; Biolegend), anti-CD8a (5H10; Caltag), anti-CD4 (RM4-5; BD Pharmingen), V450-conjugated CD45.1 (A20; BD Biosciences), PE-Cy7 conjugated anti-CD45.2 (104; BD Biosciences) and anti-CD25 (Clone PC61; BD Pharmingen). Functional grade anti-CD3 (17A2) and anti-CD28 (37.51) were purchased from eBioscience. For FACS cells were blocked with anti-mouse FcgRIII/IIR (BD Pharmingen) for 10 m and then stained for 45 m on ice with fluorophore-conjugated antibody. Stained cells were washed, fixed with 3% paraformaldehyde and analyzed on LSRII instrument with CellQuest (BD Biosciences) software. Data analyses were performed with FlowJo software (version 8.73; Tree Star).
Serum CTX and TRACP5B measurements
Food was withdrawn for 6 to 10 h prior to bleeding. Blood (100 to 200 μL) obtained via sub-mandibular vein, was allowed to clot for 2 hours at room temperature and serum collected by spinning down the cell pellet. Serum C-terminal telopeptide of type 1 collagen (serum CTX), and TRACP-5b were measured using ELISA according to the manufacturer’s instructions (Immunodiagnostic Systems, Plc.)
Matrix dissolution assays
For in-situ differentiated TcREG, on day 0 mature OC (5 × 105) were seeded on 24-well hydroxyapatite coated plates (Corning). M-CSF and GST-RANKL were added every 48 h. OC were washed and then CD8NLC or CD8Sf T-cells previously cultured with anti-CD3 and OC for 48 h were added at a 1:1 OC to T-cell ratio. On day 5, cells were removed with 10% bleach and pit area was photographed and area quantified using NIH ImageJ. The data is presented as normalized area resorbed: pitted area in treated wells divided by pitted area in untreated well. Two to three experiments for each treatment or condition were performed, where each experiment consisted of triplicate wells.
μCT data collection and analysis
The bones were scanned in μCT40 (Scanco Medical) at 55 kVp, 145 μA, and resolution of 16 μm. Gauss sigma of 1.2, Gauss support of 2, lower threshold of 237, and upper threshold of 1000 were used for all the analysis. Regions of interest were selected 50 slices below the growth plate of the proximal tibia to evaluate the trabecular compartment. Bone mineral density was obtained by quantitative μCT using Scanco Phantoms for calibration [51].
In vitro immune suppression assay
Dendritic cells (DC) were isolated from freshly harvested spleen by positive selection using anti-CD11c magnetic microbeads per manufacturer’s directions (Miltenyi). In triplicate wells: 5×104 OT-II CD4+ responder T-cells that were CFSE labeled were incubated with 5×104 DC per well in the presence of 5×104, 2.5×104, 1.25×104 and 0.625×104 OC-iTcREG. All DC were pulsed with OT-II ovalbumin peptide (OVA323-339: ISQAVHAAHAEINEAGR). Some wells were also pulsed with OT-I ovalbumin peptide (OVA257-264: SIINFEKL). The number of proliferation cycles of the responders were obtained using FlowJo proliferation platform.
Statistical Analysis
Statistical significance was assessed in all cases using paired two-tailed Mann-Whitney U test in GraphPad Prism 5.0d.
Supplementary Material
Highlights.
Transfer of CD8 T-cells with functional FoxP3 into T-cell-deficient mice limited bone loss in response to RANKL administration.
Transfer of OC-iTcREG into ovariectomized mice limited bone loss by decreasing osteoclast numbers and increased bone formation.
OC-iTcREG were also immunosuppressive in vitro and in vivo.
These studies establish a new physiological role for osteoclast-induced regulatory CD8 T-cells.
Together, our results indicate that osteoclasts regulate both the skeletal and immune system through inducing regulatory CD8 T-cells.
Acknowledgements
We thank Erin Touchette and Dr. Cheri West for expert assistance with mice and ovariectomy. We thank Dr. Steve Teitelbaum for providing the GST-RANKL bacterial expression system, as well as for discussion and advice. We also thank Sheri Koehm and Joy Eslick in the flow cytometry core. Crystal Idleburg is acknowledged for preparing slides for histomorphometry. Washington University Musculoskeletal Research Center (NIH P30 AR057235) provided partial support for these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: All authors state that they have no conflicts of interest.
Author Contributions: R.A. and Z.B. designed the experiments with advice from D.V.N. and R.D.P. Experiments were carried out by Z.B., J.K. and R.A. The data from readouts of bone histomorphometry slides and μCT was collected and analyzed by C.Y. who was blinded to samples and treatments. Z.B., D.V.N., R.D.P. and R.A. interpreted the experiments. R.A. and Z.B. wrote the manuscript.
References
- 1.Del Fattore A, Teti A, Rucci N. Bone cells and the mechanisms of bone remodelling. Front Biosci (Elite Ed) 2012;4:2302–2321. doi: 10.2741/543. [DOI] [PubMed] [Google Scholar]
- 2.Henriksen K, Neutzsky-Wulff AV, Bonewald LF, Karsdal MA. Local communication on and within bone controls bone remodeling. Bone. 2009;44:1026–1033. doi: 10.1016/j.bone.2009.03.671. [DOI] [PubMed] [Google Scholar]
- 3.Dallas SL, Rosser JL, Mundy GR, Bonewald LF. Proteolysis of latent transforming growth factor-beta (TGF-beta)-binding protein-1 by osteoclasts. A cellular mechanism for release of TGF-beta from bone matrix. J Biol Chem. 2002;277:21352–21360. doi: 10.1074/jbc.M111663200. [DOI] [PubMed] [Google Scholar]
- 4.Karsenty G, Ferron M. The contribution of bone to whole-organism physiology. Nature. 2012;481:314–320. doi: 10.1038/nature10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arron JR, Choi Y. Bone versus immune system. Nature. 2000;408:535–536. doi: 10.1038/35046196. [DOI] [PubMed] [Google Scholar]
- 6.Takayanagi H. New developments in osteoimmunology. Nat Rev Rheumatol. 2012;8:684–689. doi: 10.1038/nrrheum.2012.167. [DOI] [PubMed] [Google Scholar]
- 7.Pacifici R. Role of T cells in ovariectomy induced bone loss--revisited. J Bone Miner Res. 2012;27:231–239. doi: 10.1002/jbmr.1500. [DOI] [PubMed] [Google Scholar]
- 8.Shevach EM. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity. 2006;25:195–201. doi: 10.1016/j.immuni.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Sakaguchi S, Wing K, Yamaguchi T. Dynamics of peripheral tolerance and immune regulation mediated by Treg. Eur J Immunol. 2009;39:2331–2336. doi: 10.1002/eji.200939688. [DOI] [PubMed] [Google Scholar]
- 10.Zaiss MM, Frey B, Hess A, Zwerina J, Luther J, Nimmerjahn F, Engelke K, Kollias G, Hunig T, Schett G, David JP. Regulatory T cells protect from local and systemic bone destruction in arthritis. J Immunol. 2010;184:7238–7246. doi: 10.4049/jimmunol.0903841. [DOI] [PubMed] [Google Scholar]
- 11.Buchwald ZS, Kiesel JR, DiPaolo R, Pagadala MS, Aurora R. Osteoclast Activated FoxP3(+) CD8(+) T-Cells Suppress Bone Resorption in vitro. PLoS ONE. 2012;7:e38199. doi: 10.1371/journal.pone.0038199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiesel J, Miller C, Abu-Amer Y, Aurora R. Systems level analysis of osteoclastogenesis reveals intrinsic and extrinsic regulatory interactions. Dev Dyn. 2007;236:2181–2197. doi: 10.1002/dvdy.21206. [DOI] [PubMed] [Google Scholar]
- 13.Kiesel JR, Buchwald ZS, Aurora R. Cross-presentation by osteoclasts induces FoxP3 in CD8+ T cells. J Immunol. 2009;182:5477–5487. doi: 10.4049/jimmunol.0803897. [DOI] [PubMed] [Google Scholar]
- 14.Choi Y, Woo KM, Ko SH, Lee YJ, Park SJ, Kim HM, Kwon BS. Osteoclastogenesis is enhanced by activated B cells but suppressed by activated CD8(+) T cells. Eur J Immunol. 2001;31:2179–2188. doi: 10.1002/1521-4141(200107)31:7<2179::aid-immu2179>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 15.John V, Hock JM, Short LL, Glasebrook AL, Galvin RJ. A role for CD8+ T lymphocytes in osteoclast differentiation in vitro. Endocrinology. 1996;137:2457–2463. doi: 10.1210/endo.137.6.8641199. [DOI] [PubMed] [Google Scholar]
- 16.Grcevic D, Lee SK, Marusic A, Lorenzo JA. Depletion of CD4 and CD8 T lymphocytes in mice in vivo enhances 1,25-dihydroxyvitamin D3-stimulated osteoclast-like cell formation in vitro by a mechanism that is dependent on prostaglandin synthesis. J Immunol. 2000;165:4231–4238. doi: 10.4049/jimmunol.165.8.4231. [DOI] [PubMed] [Google Scholar]
- 17.Toraldo G, Roggia C, Qian WP, Pacifici R, Weitzmann MN. IL-7 induces bone loss in vivo by induction of receptor activator of nuclear factor kappa B ligand and tumor necrosis factor alpha from T cells. Proc Natl Acad Sci U S A. 2003;100:125–130. doi: 10.1073/pnas.0136772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weitzmann MN, Pacifici R. The role of T lymphocytes in bone metabolism. Immunol Rev. 2005;208:154–168. doi: 10.1111/j.0105-2896.2005.00324.x. [DOI] [PubMed] [Google Scholar]
- 19.Tomimori Y, Mori K, Koide M, Nakamichi Y, Ninomiya T, Udagawa N, Yasuda H. Evaluation of Pharmaceuticals With a Novel Fifty-Hour Animal Model of Bone Loss *. J Bone Miner Res. 2009 doi: 10.1359/jbmr.090217. [DOI] [PubMed] [Google Scholar]
- 20.Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Min B, Yamane H, Hu-Li J, Paul WE. Spontaneous and homeostatic proliferation of CD4 T cells are regulated by different mechanisms. J Immunol. 2005;174:6039–6044. doi: 10.4049/jimmunol.174.10.6039. [DOI] [PubMed] [Google Scholar]
- 22.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 23.Godfrey VL, Wilkinson JE, Rinchik EM, Russell LB. Fatal lymphoreticular disease in the scurfy (sf) mouse requires T cells that mature in a sf thymic environment: potential model for thymic education. Proc Natl Acad Sci U S A. 1991;88:5528–5532. doi: 10.1073/pnas.88.13.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou X, Bailey-Bucktrout S, Jeker LT, Bluestone JA. Plasticity of CD4(+) FoxP3(+) T cells. Curr Opin Immunol. 2009;21:281–285. doi: 10.1016/j.coi.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deselm CJ, Takahata Y, Warren J, Chappel JC, Khan T, Li X, Liu C, Choi Y, Kim YF, Zou W, Teitelbaum SL. IL-17 mediates estrogen-deficient osteoporosis in an Act1-dependent manner. J Cell Biochem. 2012;113:2895–2902. doi: 10.1002/jcb.24165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klinck J, Boyd SK. The magnitude and rate of bone loss in ovariectomized mice differs among inbred strains as determined by longitudinal in vivo micro-computed tomography. Calcif Tissue Int. 2008;83:70–79. doi: 10.1007/s00223-008-9150-5. [DOI] [PubMed] [Google Scholar]
- 27.Krum SA, Miranda-Carboni GA, Hauschka PV, Carroll JS, Lane TF, Freedman LP, Brown M. Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival. EMBO J. 2008;27:535–545. doi: 10.1038/sj.emboj.7601984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rissanen JP, Suominen MI, Peng Z, Halleen JM. Secreted tartrate-resistant acid phosphatase 5b is a Marker of osteoclast number in human osteoclast cultures and the rat ovariectomy model. Calcif Tissue Int. 2008;82:108–115. doi: 10.1007/s00223-007-9091-4. [DOI] [PubMed] [Google Scholar]
- 29.Lee SK, Kadono Y, Okada F, Jacquin C, Koczon-Jaremko B, Gronowicz G, Adams DJ, Aguila HL, Choi Y, Lorenzo JA. T lymphocyte-deficient mice lose trabecular bone mass with ovariectomy. J Bone Miner Res. 2006;21:1704–1712. doi: 10.1359/jbmr.060726. [DOI] [PubMed] [Google Scholar]
- 30.Robbie-Ryan M, Pacifici R, Weitzmann MN. IL-7 drives T cell-mediated bone loss following ovariectomy. Ann N Y Acad Sci. 2006;1068:348–351. doi: 10.1196/annals.1346.051. [DOI] [PubMed] [Google Scholar]
- 31.Reszka AA, Rodan GA. Bisphosphonate mechanism of action. Curr Rheumatol Rep. 2003;5:65–74. doi: 10.1007/s11926-003-0085-6. [DOI] [PubMed] [Google Scholar]
- 32.Matzelle MM, Gallant MA, Condon KW, Walsh NC, Manning CA, Stein GS, Lian JB, Burr DB, Gravallese EM. Resolution of inflammation induces osteoblast function and regulates the Wnt signaling pathway. Arthritis Rheum. 2012;64:1540–1550. doi: 10.1002/art.33504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walsh NC, Reinwald S, Manning CA, Condon KW, Iwata K, Burr DB, Gravallese EM. Osteoblast function is compromised at sites of focal bone erosion in inflammatory arthritis. J Bone Miner Res. 2009;24:1572–1585. doi: 10.1359/jbmr.090320. [DOI] [PubMed] [Google Scholar]
- 34.Tang Q, Bluestone JA, Kang SM. CD4(+)Foxp3(+) regulatory T cell therapy in transplantation. J Mol Cell Biol. 2012;4:11–21. doi: 10.1093/jmcb/mjr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blander JM. Signalling and phagocytosis in the orchestration of host defence. Cell Microbiol. 2007;9:290–299. doi: 10.1111/j.1462-5822.2006.00864.x. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki M, Jagger AL, Konya C, Shimojima Y, Pryshchep S, Goronzy JJ, Weyand CM. CD8+CD45RA+CCR7+FOXP3+ T Cells with Immunosuppressive Properties: A Novel Subset of Inducible Human Regulatory T Cells. J Immunol. 2012;189:2118–2130. doi: 10.4049/jimmunol.1200122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lerret NM, Houlihan JL, Kheradmand T, Pothoven KL, Zhang ZJ, Luo X. Donor-Specific CD8(+) Foxp3(+) T Cells Protect Skin Allografts and Facilitate Induction of Conventional CD4(+) Foxp3(+) Regulatory T Cells. Am J Transplant. 2012;12:2335–2347. doi: 10.1111/j.1600-6143.2012.04120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robb RJ, Lineburg KE, Kuns RD, Wilson YA, Raffelt NC, Olver SD, Varelias A, Alexander KA, Teal BE, Sparwasser T, Hammerling GJ, Markey KA, Koyama M, Clouston AD, Engwerda CR, Hill GR, MacDonald KP. Identification and expansion of highly suppressive CD8(+)FoxP3(+) regulatory T cells after experimental allogeneic bone marrow transplantation. Blood. 2012;119:5898–5908. doi: 10.1182/blood-2011-12-396119. [DOI] [PubMed] [Google Scholar]
- 40.Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003;423:349–355. doi: 10.1038/nature01660. [DOI] [PubMed] [Google Scholar]
- 41.Lanyon L, Sugiyama T, Price J. Regulation of bone mass: Local control or systemic influence or both? IBMS BoneKEy. 2009;6:218–226. [Google Scholar]
- 42.Munsky B, Neuert G, van Oudenaarden A. Using gene expression noise to understand gene regulation. Science. 2012;336:183–187. doi: 10.1126/science.1216379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alberts B, Wilson JH, Hunt T. Molecular biology of the cell. 5th ed. New York: Garland Science; 2008. [Google Scholar]
- 44.Lymperi S, Ersek A, Ferraro F, Dazzi F, Horwood NJ. Inhibition of osteoclast function reduces hematopoietic stem cell numbers in vivo. Blood. 2011;117:1540–1549. doi: 10.1182/blood-2010-05-282855. [DOI] [PubMed] [Google Scholar]
- 45.Morimoto R, Uehara S, Yatsushiro S, Juge N, Hua Z, Senoh S, Echigo N, Hayashi M, Mizoguchi T, Ninomiya T, Udagawa N, Omote H, Yamamoto A, Edwards RH, Moriyama Y. Secretion of Lglutamate from osteoclasts through transcytosis. Embo J. 2006;25:4175–4186. doi: 10.1038/sj.emboj.7601317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schreiweis MA, Butler JP, Kulkarni NH, Knierman MD, Higgs RE, Halladay DL, Onyia JE, Hale JE. A proteomic analysis of adult rat bone reveals the presence of cartilage/chondrocyte markers. J Cell Biochem. 2007;101:466–476. doi: 10.1002/jcb.21196. [DOI] [PubMed] [Google Scholar]
- 47.Gravallese EM. Bone destruction in arthritis. Ann Rheum Dis. 2002;61(Suppl 2):ii84–ii86. doi: 10.1136/ard.61.suppl_2.ii84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tada Y, Ho A, Koh DR, Mak TW. Collagen-induced arthritis in CD4- or CD8-deficient mice: CD8+ T cells play a role in initiation and regulate recovery phase of collagen-induced arthritis. J Immunol. 1996;156:4520–4526. [PubMed] [Google Scholar]
- 49.Borza DB. Autoepitopes and alloepitopes of type IV collagen: role in the molecular pathogenesis of anti-GBM antibody glomerulonephritis. Nephron Exp Nephrol. 2007;106:e37–e43. doi: 10.1159/000101791. [DOI] [PubMed] [Google Scholar]
- 50.Huter EN, Punkosdy GA, Glass DD, Cheng LI, Ward JM, Shevach EM. TGF-beta-induced Foxp3+ regulatory T cells rescue scurfy mice. Eur J Immunol. 2008;38:1814–1821. doi: 10.1002/eji.200838346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nazarian A, Snyder BD, Zurakowski D, Muller R. Quantitative micro-computed tomography: a non-invasive method to assess equivalent bone mineral density. Bone. 2008;43:302–311. doi: 10.1016/j.bone.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 52.Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell. 2007;130:811–823. doi: 10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.