Abstract
After surgery, rodents frequently receive acetaminophen-treated drinking water for pain relief, but the effectiveness of this practice is often questioned. Gel products are now available to facilitate the delivery of oral medication to rodents after surgery. We sought to compare consumption of flavored medicated gel and medicated water after surgery and to determine whether providing supplemental acetaminophen in gel form ensures the ingestion of a therapeutic dose of an analgesic after surgery. Male C57BL/6 mice were allocated into 3 groups after surgery: those that received acetaminophen-treated water and untreated gel (MW group); those that received medicated gel and untreated water (MG group); and those that received acetaminophen in both forms (MWG group). Total water and gel consumption were monitored daily from the day before surgery until 2 d thereafter. Mice in the MG group consumed significantly less gel than water, and consequently, the total acetaminophen dose per mouse in the MG group (49 mg/kg) was significantly less than that of the MWG group (347 mg/kg). Although the dose consumed by mice in the MW group (158 mg/kg) approached the targeted acetaminophen dose of 200 mg/kg, only mice in the MWG group actually achieved the desired dose. The results of this study indicate that flavored acetaminophen-containing gel can be used in combination with medicated water to ensure that rodents ingest the targeted dose of medication.
Abbreviations: MW, medicated water; MG, medicated gel; MWG, medicated water and gel
The administration of acetaminophen in the drinking water of rodents is a frequently used method of providing analgesia after surgery or other painful procedures.2,3,7-9,12,13,17,18 The advantages of self-administration of medication via the drinking water include stress reduction due to decreased handling or restraint, no disruption of the diurnal rhythm of the rodents being treated, and no disruption of the other animals in the room.7,13,17 Although a common practice, the literature is conflicting with regard to whether the administration of an analgesic (usually acetaminophen) in the drinking water is truly effective for rodents.2,3,6,13,17,18
Several explanations have been proposed regarding why the administration of acetaminophen in drinking water may be ineffective. Water intake may be decreased after a potentially painful surgery or procedure because of the effects of stress from manipulation or ineffectively managed pain.8,11 However, rodents that underwent surgery actually drank more of the medicated water than untreated water as compared with rodents that did not undergo surgery.13
Alternatively, neophobia may reduce the consumption of acetaminophen-treated water. Neophobia (‘fear of the new’),2,13 especially to novel tastes, is commonly observed in rodents. This phenomenon leads to rodents initially consuming a small amount of an unknown substance. Although the fact that rodents exhibit neophobia is not debated, there is disagreement regarding its effect on the ingestion of medication.2,13,17 Regardless of the cause, the result is a decrease in the medicated water intake, and total consumed dose of an analgesic may be lower than its therapeutic dose, leading to insufficient analgesia.
The number of new products available to facilitate the delivery of oral medication and provide supplemental support to laboratory animals after surgery has increased recently. One such product is a flavored water gel specifically designed to deliver medication to rodents. Our institution is among many that have frequently used gel packs to provide hydration during transport.14 To our knowledge, there have not been any published studies to determine whether rodents exhibit neophobia to cherry-flavored gel, similar to the neophobia that has been demonstrated in rats to cherry-flavored water.2 The commercial producer of the gel indicates that the expected intake of the gel by mice is comparable to regular water. If so, then the flavored gel can be used as a replacement or supplement to medicated water to ensure the mice receive the appropriate dose of analgesic.
This study was incorporated into a currently approved experiment as a way to improve analgesia through a more efficient manner of delivery. This improvement is especially relevant in daily practice, because the method for providing analgesia is limited by the parameters being studied. By mimicking current methods, the current study sought to identify methods that work with current means of administering analgesia and alternative options needed to be pursued in subsequent studies.
Given that most of the research available on the ingestion of medication in water focuses on rats,2,3,13,17,18 not mice, there is a lack of consensus regarding whether providing acetaminophen in the water is effective. In addition, because new gel products are available for medication delivery, the purpose of this study was to assess the amounts of gel and water ingested and the resultant dosage of an analgesic provided to mice postsurgery in the context of currently used practices at this facility. We hypothesized that flavored medicated gel would be consumed at least as well as medicated water and that providing both forms of medication would increase overall consumption and therefore achieve the targeted therapeutic dose.
Materials and Methods
Animals.
C57BL/6NTac mice (Mus musculus; n = 42; male; age, 11 to 12 wk; weight: average, 24 g; range, 19.5 to 29.3 g) were obtained from Taconic Farms (Germantown, NY). In addition, C57BL/6NCr mice (Mus musculus; male; n = 8; age, 11 to 12 wk; weight: average, 28 g; range, 27.9 to 29.1 g) were obtained from the National Cancer Institute (Frederick, MD). The C57BL/6NCr mice were distributed into the MG and MWG groups (one cage per group). All mice were part of a study measuring inflammatory markers to develop novel therapies for spinal cord injury. For this particular portion of the experiment, no other experimental treatments or manipulations were performed on the mice. At set time points after surgery, the mice were euthanized and the spinal cords collected and analyzed. Acetaminophen was chosen over other analgesics because it does not possess significant antiinflammatory activity.4,5,16,19
Mice were housed in groups of either 3 or 4 (according to collaborator's experimental requirements) in static polycarbonate shoebox-type cages with filter tops (Ancare, Bellmore, NY) on rodent hardwood bedding (catalog no. 7090M, Laboratory-grade Maple SaniChips, Harlan Teklad, Madison, WI), and each cage had shredable nesting material (Nestlets, Ancare) for enrichment. Mice were provided pelleted rodent food (Harlan Teklad Rodent Diet 2018) and filtered (15 to 50 µm) tap water ad libitum. The room was kept on a 12:12-h light:dark cycle (lights on, 0600; lights off, 1800), temperature was maintained between 68 to 69 °F (20 to 20.5 °C), and relative humidity was 33% to 64%. All procedures were approved by the IACUC of the Uniformed Services University of the Health Sciences, which is AAALAC-accredited.
Sentinel mice were used to monitor the health status of the experimental animals. Every week, Swiss Webster mice were directly exposed to dirty bedding from all colony animals. Every quarter, the sentinel mice were euthanized by using CO2, and blood was collected. Serology samples were sent to Charles River Laboratories (Wilmington, MA) for ELISA (Mouse Serology Assessment Profile). In addition, tissues were submitted quarterly to the Joint Pathology Center (Silver Spring, MD) for histopathology. All sentinel mice and 25% of colony mice, chosen at random, were examined for fur mites and pinworms via hair plucking and tape tests. At the time of this study, all mice were negative for pinworms, fur mites, Sendai virus, pneumonia virus of mice, mouse hepatitis virus, minute virus of mice, mouse parvovirus, mouse norovirus, Theiler murine encephalomyelitis virus, reovirus, mouse rotavirus, lymphocytic choriomeningitis virus, ectromelia virus, mouse adenovirus, mouse pneumonitis virus, polyoma virus, and Mycoplasma pulmonis. Mice were not tested for Helicobacter spp.
Surgery.
Once mice were fully anesthetized with isoflurone–oxygen, 0.2 mL diluted bupivacaine was injected subcutaneously at the incision site. After approximately 2 to 3 min, a 3-cm skin incision was made from spinal T6 to T10, and the superficial and supraspinal muscles were retracted from the spinal processes. A laminectomy to remove the dorsal spinal column was performed at T8–T9, exposing the underlying spinal cord, but the dura was left intact. A moderate contusion injury to the spinal cord at T9 (impact, 50 kdyn) was made by using the Infinite Horizons Impactor (Precision Systems and Instrumentation, Lexington, KY).10,15 After impact, a drop of 0.25% bupivacaine diluted 1:1 with sterile saline was placed at the laminectomy site just prior to closure with bone wax. The site was then closed in layers by using 4-0 monofilament nylon suture in an interrupted pattern and finishing with skin staples, which were removed in 7 to 10 d.
The resulting spinal cord injury combined with the epidural effect of the bupivacaine produced clinical signs of impaired hindlimb movement and inability to ambulate, void urine, or sense pain below the lesion from 2 to 7 d after surgery. Mice were evaluated (urine status [that is, blood or cloudiness], self-mutilation, signs of pain or distress, hydration, wound status, and bladder size) twice daily for the first 7 to 10 d after surgery and then weekly thereafter. Any mice that manifested symptoms of pain such as lack of sensitivity or oversensitivity to stimulation, autophagia, scratching at wound site, vocalization in response to touch, or inflammation of surgical sites were excluded from the study and euthanized.
After anesthetic recovery, 3 or 4 mice (depending on the experimental group) were placed in a cage containing 2 in. paper cellulose bedding (Alpha-dri, Shepherd Specialty Papers, Kalamazoo, MI) allowing them to reach water and food despite their limited movement. Mice were monitored twice daily, and bladders were manually expressed when needed during those checks. No cage changes occurred during the 2 d after surgery.
Study design.
Mice were weighed every morning at approximately 1 h after lights on (approximately 0700), starting the day before surgery and ending 2 d afterward. Water bottles were weighed (increment, 0.1 g) on the morning before, of, and 2 d after surgery. Cages of mice were divided randomly into 3 experimental groups postsurgery: the medicated water group (MW, n = 5 cages [18 mice]) received acetaminophen in filtered tap water and untreated gel; the medicated gel group (MG; n = 4 cages [16 mice]) received acetaminophen in the gel and untreated water; and the medicated water and gel group (MWG; n = 4 cages [16 mice]) received acetaminophen in both filtered tap water and gel. Currently at this institute, oral medication typically is first provided to animals the day of surgery after recovery; to mimic and therefore assess current practices, oral medication was provided in the cage immediately after surgery. The amount consumed (increment, 0.1 g) was determined by weighing the bottles and gel cups daily the morning of and 2 d after surgery, when mice were weighed. After 2 d, the medicated water and gel cups were refilled with untreated tap water. The difference in the weights of the water and gel consumed between postsurgical days was divided by the number of mice per cage to determine the average amount of water or gel consumed in milliliters per day per mouse. The average acetaminophen dose per mouse was calculated by taking the average amount of water or gel ingested (in mL) multiplied by the concentration (water, 1.1 mg/mL; gel, 1.3 mg/mL) divided by the average weight (in g) of the mice in the cage each day multiplied by 1000 (to get mg/mL).
In a previous study,1 the overall mean of water intake varied by only 0.3 mL between strains, so we used average intake as the measure. The cited study1 indicated that using the average water intake for a group of mice of the same strain would be very close to that measured of a single subject. Therefore, to avoid having to use single housing and potential deprivation of social interaction, we used average water or gel intake as the measure of consumption.
Acetaminophen solution.
The recommended dose of oral acetaminophen is 110 to 305 mg/kg.5,6 At an anticipated total daily consumption of 4 to 6 mL per mouse,1,8 the desired concentration of the water and gel was approximately 1.1 mg/mL to achieve a dose within the recommended range (approximately 200 mg/kg). To prepare the acetaminophen-treated water, 1.6 mL (2 droppersful) of cherry-flavored acetaminophen liquid (Infants’ Pain Relief, Perrigo, Allegan, MI) was mixed with 150 mL filtered tap water. The acetaminophen contained 80 mg per 0.8 mL, resulting in the drinking water containing acetaminophen at 1.1 mg/mL. To prepare the acetaminophen-treated gel, 0.8 mL of the same acetaminophen liquid was added to each 2-oz. gel cup (MediGel Sucralose, Clear H2O, Portland, ME). The resulting concentration of medicated gel was 1.3 mg/mL. According to manufacturer's recommendation, to thoroughly mix the acetaminophen throughout the gel, the unopened gel was warmed in a water bath until the gel became a liquid; 0.8 mL acetaminophen liquid then was placed in a 1-mL syringe with an 18-gauge needle, the needle was inserted through the lid of the cup, and the acetaminophen was injected into the cup. The needle and syringe then were removed, and a piece of tape was placed over the hole made by the needle. The cup was shaken for approximately 10 s to ensure a homogeneous color (indicating even distribution of the acetaminophen) and then placed in a refrigerator to allow the gel to reform. The gel was brought to room temperature before being placed in the cage with the mice.
Statistical analysis.
Paired t test analysis was used to compare mean water consumption before and after surgery and the difference in water and gel consumption within each group. An aggregate one-way ANOVA (version 20, SPSS, Chicago, IL) followed by a Tukey multiple-comparisons procedure was used to compare mean water consumption per mouse, mean gel consumption per mouse, and mean acetaminophen dose per mouse among the 3 treatment groups. Weight differences were analyzed by using 2-way repeated-measures ANOVA followed by Tukey multiple comparisons. A P value of less than 0.05 was regarded as statistically significant.
Results
Gel intake.
The overall postsurgery average gel intake per mouse was 1.1 ± 0.3 mL (Table 1). There was no statistical difference (P = 0.517) between day 1 and day 2 gel intake per mouse (Table 2), so the data from both days were combined to get the average gel intake per mouse. The average per mouse gel intake for each group (Table 1) did not differ among the 3 treatment groups.
Table 1.
Water and gel ingestion per mouse (mL; mean ± SEM) by treatment group
| Water |
|||
| Group | Before surgery | After surgerya | Gel after surgerya |
| MW | 3.3 ± 0.6 | 3.1 ± 1.9 | 1.0 ± 0.2 |
| MG | 3.8 ± 1.1 | 4.2 ± 1.2b | 1.0 ± 0.4b |
| MWG | 3.2 ± 0.5 | 5.6 ± 3.4 | 1.3 ± 0.4 |
| Overall | 3.4 ± 0.7 | 4.2 ± 2.3c | 1.1 ± 0.3c |
| aAverage of days 1 and 2. | |||
| bSignificant (P = 0.019) difference between water and gel ingestion within the MG group. | |||
| cSignificant (P = 0.0004) difference between water and gel ingestion among all groups combined. | |||
Table 2.
Daily intake of water and gel per mouse (mean ± SEM) by treatment group
| Water |
Gel |
|||||
| Group | Before surgery | Day 1 after surgery | Day 2 after surgery | Day 1 after surgery | Day 2 after surgery | |
| MW | 3.3 ± 0.6 | 1.7 ± 0.9 | 4.6 ± 2.8 | 1.0 ± 0.3 | 1.0 ± 0.4 | |
| MG | 3.8 ± 1.1 | 2.8 ± 0.7 | 5.5 ± 2.4 | 1.0 ± 0.2 | 1.1 ± 0.5 | |
| MWG | 3.2 ± 0.5 | 4.9 ± 3.4 | 6.2 ± 2.5 | 1.4 ± 0.4 | 1.1 ± 0.3 | |
| Overall | 3.4 ± 0.7 | 3.1 ± 1.3 | 5.4 ± 0.7 | 1.1 ± 0.2 | 1.1 ± 0.1 | |
In the small pilot study of naïve mice, the amount of gel ingested did not differ significantly (P = 0.065) between the plain (2.7 ± 0.9 mL) and medicated (2.0 ± 0.7 mL) gel.
Water intake.
The overall postsurgery average water intake per mouse was 4.2 ± 2.3 mL (Table 1). There was no statistical difference (P = 0.143) between day 1 and day 2 water intake per mouse (Table 2), so the data from both days were combined to get the average water intake per mouse. The average per mouse water for each group (Table 1) did not differ among the 3 treatment groups.
Average water consumption per mouse (Table 1) before surgery did not differ significantly from that after surgery.
Gel and water comparison.
Overall, mice in all treatment groups ingested significantly (P = 0.0004) more water than gel (Table 1). However, when the data were compared within each group, the difference between water and gel intake was significant (P = 0.019) only in the MG mice.
Weight.
Average weight did not differ between groups (Table 3) or between postsurgery days 1 and 2. However, within groups, weight before surgery differed (P < 0.05) from that after surgery on both days 1 and 2 (Table 3). Therefore although weight decreased over time, there were no differences between groups, either overall or at any point in time.
Table 3.
Average weight (g) of mice per treatment group
|
P |
||||||
| Group | Before surgery | Day 1 after surgery | Day 2 after surgery | Before surgery compared with day 1 afterward | Before surgery compared with day 2 afterward | Day 1 after surgery compared with day 2 afterward |
| MW | 23.1 ± 1.9 | 21.7 ± 1.6 | 21.8 ± 1.3 | 0.0002 | 0.0005 | 0.9588 |
| MG | 24.8 ± 2.5 | 23.2 ± 2.6 | 23.5 ± 2.5 | 0.0003 | 0.0022 | 0.6768 |
| MWG | 24.4 ± 2.9 | 22.8 ± 2.5 | 22.6 ± 2.4 | 0.0002 | 0.0001 | 0.8521 |
Significant P values (that is, P < 0.05) are indicated in bold type.
Dose.
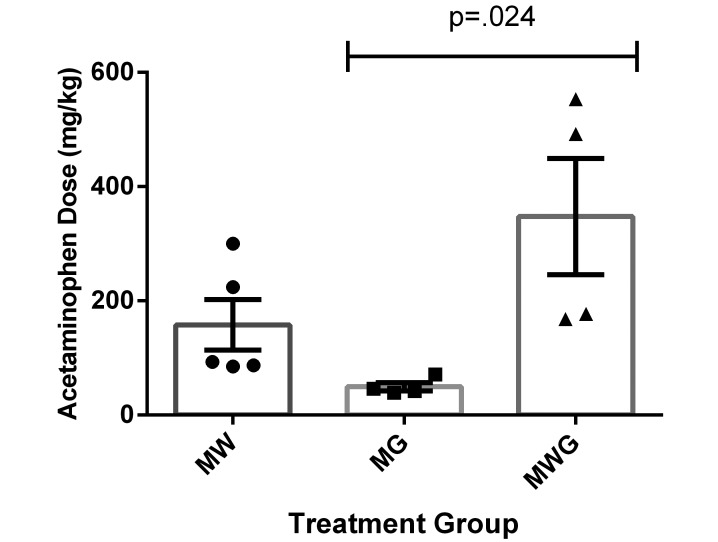
Mice in the MG group ingested the lowest dose of acetaminophen (49 mg/kg), whereas mice in the MWG group ingested the highest dose (347 mg/kg), leading to a significant difference (P = 0.024) in average acetaminophen dose per mouse between MG and MWG mice (Figure 1). The average acetaminophen dose for mice from the MW group (158 mg/kg) did not differ significantly from those of the other 2 groups.
Figure 1.
Average acetaminophen dose per mouse according to water and gel consumption (mg/kg; mean ± SEM) in the MW (circles), MG (squares), and MWG (triangles) groups respectively. There was a significant difference between the MG and MWG groups only.
Activity level.
Activity level and ability to ambulate throughout the cage were observed and demonstrated no apparent difference in activity level between groups. All mice were bright, alert, and active by the following morning, approximately 15 h after surgery. One of the expected side effects of this particular procedure was limited use of one or both of the hindlimbs. Even with the difficulty to ambulate on their hindlimbs, the mice were observed to move around the cage and reach both the gel (which was on the cage bottom, with the opening even with the top of the bedding) and the water bottle (which hung from the wire top). Only one mouse (MWG group) was removed from the study (due to complications from the incision [removed skin staples]) and euthanized.
Discussion
According to information provided by the commercial producer of the gel, the expected intake of nonmedicated gel by mice is the same as water intake (4 to 6 mL daily). However, our current study found that the average gel intake of all 3 groups, regardless of whether it was medicated, was 1.1 mL daily on average. One possible explanation for this difference is water was available in the cage, subsequently reducing the intake of gel. The vendor's information did not clarify whether the mice in their study had access to only the medicated gel or in addition to water. In the MG group, the mice consumed significantly more water than gel, even though there was no analgesic in the water. In the MWG group where both vehicles were medicated, the mice still ingested more water than gel. We did not compare the consumption of unmedicated water and gel, because this experiment could be performed before surgery only, due to its lack of pain relief. Although we directly compared gel and water intakes in each cage, the results imply that mice in a potential subsequent study should receive gel only or water only to determine consumption when the vehicles are offered separately.
A second possible explanation for the difference in intake between the 2 vehicles is neophobia of the gel. Although neophobia to the gel might have been expected because of its texture or flavor, neophobia of the medicated water due to its color or flavor might also have been expected to affect its intake.2,17 However, this neophobia was not noted, thus supporting previous findings13 suggesting that the possible neophobia to acetaminophen in water does not decrease ingestion. Because the study was designed to mimic current conditions at the facility, gel was not given before surgery. Regardless, our results indicate that follow-up studies should investigate the neophobia to gel by introducing the gel 1 to 2 d before surgery.
Previous studies in rats have shown that water consumption increases after surgery,13 but the difference in water consumption between before and after surgery in our mice did not reach statistical significance. With an increase in the number of mice evaluated, a significant increase in postsurgery water consumption might emerge. In addition, in all groups, body weight before surgery was less than that on postsurgery days 1 and 2, perhaps indicating some degree of dehydration. This notion supports the theory of previous authors,13 who considered that the increase in water intake after surgery is due to the alleviation of mild dehydration rather than to alleviation of pain13 or to enhanced flavoring of the water from the cherry-flavored medication.
Although the mice were observed to access both the water and the gel, their limited ambulation may have affected how much water or gel was consumed. Water and gel consumption after surgery did not differ from that before surgery, but perhaps postsurgery consumption did not increase as much as it might have had the mice not had limited ambulation. As a result, the trend in increased consumption might have been significant if a different model had been used. We performed a small pilot study (5 cages of C57BL/6NTac mice [4 mice per cage]) to investigate water and gel consumption over 2 d in naïve mice. In the current study, mice received untreated tap water and plain gel for 2 d, followed by acetaminophen-loaded water and gel. The amount of gel ingested did not differ between the untreated and medicated gel (2.7 ± 0.9 mL and 2.0 ± 0.7 mL, respectively), but the naïve mice consumed significantly (P = 0.01) more medicated gel than did those that had undergone surgery (2.0 ± 0.7 mL and 1.1 ± 0.3 mL, respectively). This finding suggests that even though the postsurgical mice were observed to consume the gel, that consumption was reduced after surgery. It also indicates that oral analgesics may have varying degrees of efficacy depending on the type of surgery or manipulations performed.
The focus of the current study was not to determine whether acetaminophen provides sufficient postsurgical pain relief, because there already have been several studies that deal with that topic in both mice and rats.3,12,13,17,18 Instead, our focus was to establish whether the mice were ingesting enough of acetaminophen-treated water or gel to achieve the dose expected to provide pain relief. According to our 200-mg/kg threshold, the mice in the MW (158 mg/kg) and MG (49 mg/kg) groups did not ingest enough acetaminophen to reach the targeted therapeutic dose. The MW dose was substantially lower than the average dose per mouse of the MWG group (347 mg/kg). Mice in the MG group had the lowest dose of all 3 groups—a dose that was significantly lower that of the MWG group. Given that the average gel intake was lower than expected, it is understandable that the average dose of acetaminophen was also low, given that the mice had access to acetaminophen only in gel and not water. Conversely, the mice that had access to acetaminophen in both water and gel had the largest acetaminophen dose. This outcome might be expected, given that the mice in the MWG group had twice the opportunity to ingest acetaminophen, whereas the other 2 groups had it available in only one vehicle. The mice in the MWG group, although they achieved the therapeutic dose, also had high levels of consumption that may be approaching toxic levels.12 Given that all of our data are based on average acetaminophen dose per mouse, it should be noted that individual doses may have varied, because the mice were group-housed. In addition, the variability of when and how often the mice ingested the acetaminophen may have affected the total dose. So whereas some mice in the MWG may have appeared to ingest toxic levels of acetaminophen, the fact that they ingested it over the span of 24 h and not all at one time lessens the concern that toxicity could be an issue. The variability between the amount consumed and total dose is one of the inherent concerns with providing analgesia in drinking water, and the results of the current study prompt caution about the efficacy of self-medication.
Another concern regarding the administration of medication in drinking water is that mice tend to consume water at night, perhaps leading to subtherapeutic levels during the day. However, other colleagues13 demonstrated reliable analgesia within 3 to 4 h of the end of the dark cycle. This finding may indicate that considerable amounts of drinking occurred during the early morning hours, given that the half-life of acetaminophen in plasma is about 2 h.13 Although the cited study12 was done in rats and not mice, mice follow the same drinking habits as rats, and the same principle can be applied to mice. This finding does not guarantee that therapeutic levels will last throughout the entire day, but they may last longer than initially expected.
Mice in all 3 groups ingested the same amount of gel regardless of whether it was medicated or not. This pattern led to a very tight data set in the MG group. Because the consumption of the gel did not significantly differ among the groups, it is unlikely that the cherry flavor, which was present only in the medicated gel, affected consumption of the gel. However, average water consumption, either medicated or untreated, varied among all 3 groups more than we expected. Because water ingestion varied more than did gel ingestion, the subsequent doses of groups with acetaminophen in the water varied more than did those of the MG group, which had acetaminophen only in the gel. Interestingly, in our pilot study, naïve mice exposed to untreated or medicated water over 2 d drank significantly (P = 0.009) more medicated than untreated water (1.8 ± 0.6 mL and 3.3 ± 1.3 mL, respectively). Because the naïve mice did not undergo any surgical manipulation, these results suggest that perhaps the flavoring has a greater effect on increasing ingestion than we initially thought.
In summary, the findings of the current study did not support our hypothesis that flavored medicated gel would be consumed at least as well as medicated water. As a result, the mice that had access to medicated gel only did not ingest enough at the prepared concentration to achieve the targeted dose of analgesic after surgery. However, our hypothesis regarding providing medicated gel in conjunction with medicated water was validated, and this practice appears to be an optimal method to ensure that mice receive the correct therapeutic dose of the desired medication. Given the increased attention to providing appropriate pain relief in the current research environment, this strategy provides a simple yet effective way to supplement oral delivery options of medications in mice.
Acknowledgments
Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Uniformed Services University or the Department of Defense. We thank Dr Cara Olsen for statistical analysis and consultation and Ms Guzal Khayrullina for technical assistance. This work was funded in part by the NINDS/NIH (Grant number 1R01NS073667-01A1, KB).
References
- 1.Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. 2002. Food intake, water intake, and drinking-spout side preference of 28 mouse strains. Behav Genet 32:435–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer DJ, Christenson TJ, Clark KR, Powell SK, Swain RA. 2003. Acetaminophen as a postsurgical analgesic in rats: a practical solution to neophobia. Contemp Top Lab Anim Sci 42:20–25 [PubMed] [Google Scholar]
- 3.Cooper DM, DeLong D, Gillett CS. 1997. Analgesic efficacy of acetaminophen and buprenorphine administered in the drinking water of rats. Contemp Top Lab Anim Sci 36:58–62 [PubMed] [Google Scholar]
- 4.Cotroneo TM, Hugunin KM, Shuster KA, Hwang HJ, Kakaraparthi BN, Nemzek-Hamlin JA. 2012. Effects of buprenorphine on a cecal ligation and puncture model in C57BL/6 mice. J Am Assoc Lab Anim Sci 51:357–365 [PMC free article] [PubMed] [Google Scholar]
- 5.Fish RE, Brown MJ, Danneman PJ, Karas AZ, editors. 2008. Anesthesia and analgesia in laboratory animals, 2nd ed. New York (NY): Elsevier. [Google Scholar]
- 6.Flecknell PA. 1984. The relief of pain in laboratory animals. Lab Anim 18:147–160 [DOI] [PubMed] [Google Scholar]
- 7.Flecknell PA. 1996. Laboratory animal anesthesia. London (UK): Academic Press. [Google Scholar]
- 8.Fox JG, Anderson LC, Loew FM, Quimby FW, editors. 2002. Laboratory animal medicine, 2nd ed. San Diego (CA): Academic Press. [Google Scholar]
- 9.Kolstad AM, Rodriguiz RM, Kim CJ, Hale LP. 2012. Effect of pain management on immunization efficacy in mice. J Am Assoc Lab Anim Sci 51:448–457 [PMC free article] [PubMed] [Google Scholar]
- 10.Lee HJ, Wu J, Chung J, Wrathall JR. 2013. SOX2 expression is upregulated in adult spinal cord after contusion injury in both oligodendrocyte lineage and ependymal cells. J Neurosci Res 91:196–210 [DOI] [PubMed] [Google Scholar]
- 11.Liles JH, Flecknell PA. 1993. The effects of surgical stimulus on the rat and the influence of analgesic treatment. Br Vet J 149:515–525 [DOI] [PubMed] [Google Scholar]
- 12.Matsumiya LC, Sorge RE, Sotocinal SG, Tabaka JM, Wieskopf JS, Zaloum A, King OD, Mogil JS. 2012. Using the mouse grimace scale to reevaluate the efficacy of postoperative analgesics in laboratory mice. J Am Assoc Lab Anim Sci 51:42–49 [PMC free article] [PubMed] [Google Scholar]
- 13.Mickley GA, Hoxha Z, Biada JM, Kenmuir CL, Bacik SE. 2006. Acetaminophen self-administered in the drinking water increases the pain threshold of rats (Rattus norvegicus). J Am Assoc Lab Anim Sci 45:48–54 [PubMed] [Google Scholar]
- 14.Moccia KD, Olsen CH, Mitchell JM, Landauer MR. 2010. Evaluation of hydration and nutritional gels as supportive care after total-body irradiation in mice (Mus musculus). J Am Assoc Lab Anim Sci 49:323–328 [PMC free article] [PubMed] [Google Scholar]
- 15.Pajoohesh-Ganji A, Knoblach SM, Faden AI, Byrnes KR. 2012. Characterization of inflammatory gene expression and galectin 3 function after spinal cord injury in mice. Brain Res 1475:96–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plumb DC. 2008. Veterinary drug handbook. Oxford (UK): Wiley-Blackwell. [Google Scholar]
- 17.Speth RC, Smith MS, Brogan RS. 2001. Regarding the inadvisability of administering postoperative analgesics in the drinking water of rats (Rattus norvegicus). Contemp Top Lab Anim Sci 40:15–17 [PubMed] [Google Scholar]
- 18.St A Stewart L, Martin WJ. 2003. Evaluation of postoperative analgesia in a rat model of incisional pain. Contemp Top Lab Anim Sci 42:28–34 [PubMed] [Google Scholar]
- 19.Volker DB, Gentle R, Garg M. 2000. Oral buprenorphine is antiinflammatory and modulates the pathogenesis of streptococcal cell wall polymer-induced arthritis in the Lew/SSN rat. Lab Anim 34:423–429 [DOI] [PubMed] [Google Scholar]