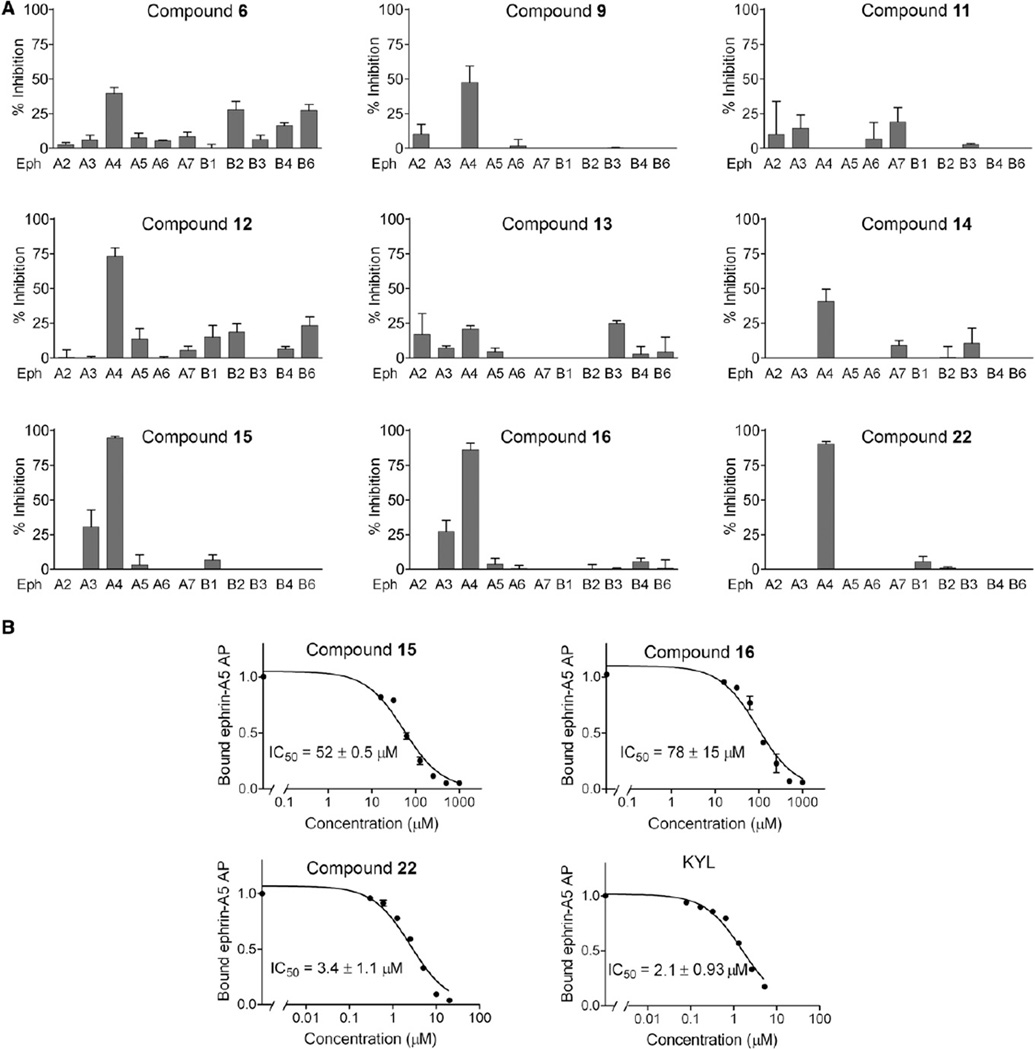
Figure 6. Compounds Identified by the HTS by NMR Inhibit Ephrin Ligand Binding to EphA4 and Ephrin-Dependent EphA4 Tyrosine Phosphorylation in Cells.
(A) Inhibition of ephrin-A5 AP binding to immobilized EphA receptor Fc fusion proteins and ephrin-B2 AP binding to immobilized EphB receptor Fc fusion proteins in the presence of the peptides relative to no peptide. All the compounds were tested at 500 µM, with the exception of compound 22, which was tested at 15 µM. Error bars represent the standard errors from two to four measurements.
(B) Inhibition of ephrin-A5 AP binding to immobilized EphA4 Fc by compounds 15, 16, and 22 and by the KYL peptide. Error bars represent the standard errors from two to four measurements. R2 values indicating the goodness of fit for the inhibition curves were 0.97 for peptide 15, 0.95 for peptide 16, 0.93 for peptide 22 and 0.97 for KYL.
See also Figures S5–S7 and Table S4.