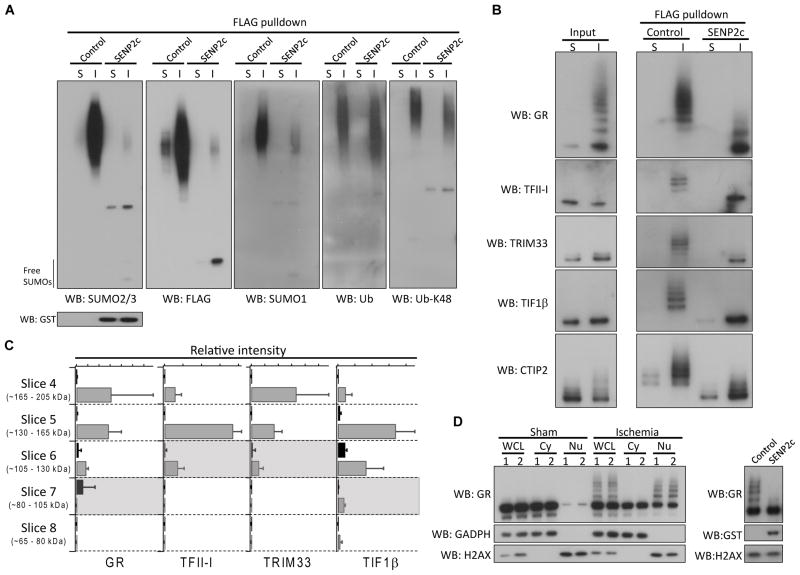
Figure 5.
Verification of SUMO3 proteomics data. A–B, The FLAG pulldown samples (sham and ischemia) treated with/without SENP2c were analyzed by Western blotting with the indicated antibodies. SENP2c is GST tagged. C, Quantitative analysis of 4 identified SUMO3 substrates. The areas under the curve of two peptides from each protein were used to perform relative quantitation of sham (black) and ischemia (grey) samples as a function of molecular weight. The expected molecular weight of each unmodified protein was indicated by a shaded region. Data were presented as means ± SD (n = 3 per group). D, Whole cell lysates (WCL), cytoplasmic (Cy) and nuclear (Nu) fractions, prepared from cortical samples (n = 2/group, sham and ischemia) were analyzed by Western blotting (left panel) to evaluate SUMOylation of glucocorticoid receptor (GR). To confirm that slow migrating bands were indeed SUMOylated forms of GR, a post-ischemic nuclear fraction was treated with/without SENP2c. SENP2c treatment abolished almost all slow migrating bands (right panel). GADPH and H2AX were used as cytoplasmic and nuclear markers, respectively. S, sham; I, ischemia; WB, Western blot.; Ub, ubiquitin.