Coffee is one of the most consumed and popular beverages in the world nowadays. According to the National Coffee Association, more than 60% of American adults drink coffee each day, and an average of 3.1 cups of coffee are consumed by each drinker per day. In spite of its popularity, it has been controversial whether drinking coffee is detrimental or beneficial for human health. While a large body of evidence suggests that drinking coffee may be beneficial for a variety of chronic health conditions including type 2 diabetes, stroke, cancer and all-cause mortality, some other studies suggest that drinking coffee may be a potential hazard for coronary heart disease and may increase mortality in younger drinkers less than 55 years old. 1,2 The reasons for the conflicting results from these large population-based studies could be very complex, but one possibility could be due to the remarkable variety of different types of coffee and the preparation and brewing methods around the world. In contrast to the controversy regarding the health effect of coffee on other organs and tissues, all the experimental and population-based studies support unanimous beneficial effects of drinking coffee on the liver.
The early evidence of the beneficial effects of coffee on the liver came from epidemiologic studies that revealed a strong association of drinking coffee with decreased serum hepatic enzymes including gamma-glutamyltransferase, aspartate aminotransferase and alanine aminotransferase in persons with high risk of liver injury such as alcoholic, diabetic and viral infection.3 Recent epidemiologic studies further support that drinking coffee also reduces the risk for fatty liver, fibrosis and hepatocellular carcinoma. 4, 5
While epidemiological evidence strongly supports the beneficial effects of coffee on liver functions, the molecular mechanisms for its actions are less understood. Part of the reasons may be because coffee contains a number of different contents including caffeine, diterpenoid alcohols cafestol and kahweol, and other antioxidant substances such as chlorogenic acid and tocopherols. Coffee may increase antioxidant activity to offer hepato-protective action by directly activating Nrf2 (nuclear factor erythroid 2-related factor) transcription factor or indirectly increasing the expression of UDP glucuronosyltransferase in hepatocytes.6 Caffeine, the major component of coffee, is metabolized mainly in the liver via cytochrome P450 1A2, which generates three metabolic dimethylxanthines including paraxanthine (84%), theobromine (12%) and theophyline.7 It is well known that methylxanthines increase intracellular cAMP levels by inhibiting phosphodiesterase activity. Indeed, caffeine increased intracellular cAMP levels in hepatocytes. As a result, caffeine inhibited liver fibrosis by down-regulation of connective tissue growth factor (CTGF), an important player for fibrosis mediated by transforming growth factor β (TGF-β). Mechanistically, it was found that caffeine promoted proteasomal degradation of the TGF-β effector protein Smad2.7, 8 Furthermore, coffee might also reduce hepatic lipid accumulation by increasing fatty acid β-oxidation and reducing liver oxidative stress and inflammation, as suggested by a rat model of steatohepatitis.9
Autophagy is an intracellular degradation pathway that involves the formation of a double-membrane autophagosome, which enwraps and delivers cargo to lysosome where the contents are degraded. Autophagy is usually activated as a catabolic process when cells lack nutrients and energy. Autophagy was initially thought to be a bulk non-selective degradation pathway for degrading intracellular proteins and excess/or damaged organelles. However, a pioneer work from Singh et al. demonstrates that autophagy can selectively degrade intracellular lipid droplets (LDs), a process which is termed lipophagy.10 Ever since then, many follow up studies including ours have demonstrated that pharmacologically modulating autophagy can attenuate both alcoholic and non-alcoholic steatosis in mouse livers.11, 12 LDs are organelles enriched with triglycerides and cholesterol esters that are surrounded by a phospholipid monolayer as lipid stores for future use or to detoxify the otherwise toxic free fatty acids (FFAs). When more energy is needed or too much influx of lipids occurs, cells activate the lipolysis process mediated by intracellular lipases to generate FFAs. In addition to the hydrolases such as proteases, glycases and nucleases, the lysosome also contains acid lipases (low pH is required for their maximal enzymatic activity).13 Currently, it is not clear how cytosolic lipases and lysosomes coordinately decide the amount and type of lipids to be degraded. At normal physiological conditions, it is thought that lysosome-mediated lipid degradation is mainly responsible for membranes of organelles or extracellular lipids that reach lysosomes from endocytosis. However, when cells are overloaded with lipids, lipophagy may be able to provide a large amount of FFAs within a short time period by degrading cellular LDs.13 It is well known that FFAs are toxic to cells and thus, too much FFAs generated from lipophagy/lipolysis may harm the cell unless FFAs are quickly cleared. Cells can utilize mitochondrial β-oxidation to burn FFAs to generate ATP and in turn reduce their toxicities. Therefore, an ideal intervention for treating fatty liver disease would be to enhance not only lipophagy but also the use of FFAs.
In this issue of Hepatology, Sinha et al. report on a study demonstrating that caffeine may be such an ideal intervention to protect against fatty liver diseases by enhancing lipophagy and mitochondrial-β oxidation simultaneously.14 By using a series of autophagic flux assays, they demonstrated that caffeine induces autophagic flux in human hepatoma cells, primary cultured hepatocytes and mouse livers. They further found that caffeine-induced autophagosomes often contain lipid droplets, suggesting the induction of lipophagy. Metabolomic analysis of hepatic acylcarnitines revealed an increase of hepatic lipolysis by caffeine treatment. More importantly, caffeine increased mitochondrial β-oxidation activity and inhibited hepatic steatosis in a high fat diet-fed mouse model. Interestingly, they found that siRNA knockdown of Atg5, an essential autophagy gene that is required for the biogenesis of autophagosomes, inhibited caffeine-induced mitochondrial β-oxidation and mitochondrial bioenergetics. Selective removal of damaged mitochondria by mitophagy may help to maintain better quality of mitochondria and thus enhance the mitochondrial β-oxidation and mitochondrial bioenergetics. Unfortunately, potential changes of mitophagy after caffeine treatment were not addressed in the current study.
How does caffeine trigger autophagy in hepatocytes? The mammalian target of rapamycin complex 1 (mTORC1) and the AMP-activated protein kinase (AMPK) are two important regulators for autophagy, which are also two key sensors in response to the changes of cellular nutrients and energy. mTORC1 negatively regulates autophagy by inhibiting ULK1 activity via direct phosphorylating ULK1.15 In contrast, AMPK positively regulates autophagy by at least two mechanisms. AMPK suppresses mTORC1 activity by phosphorylation of TSC2 and raptor, two essential regulators of mTORC1,16, 17 and AMPK activates ULK1 complex by directly phosphorylating ULK1 at different sites than mTORC1.15, 18
Indeed, caffeine treatment decreased mTORC1 activity both in cultured hepatocytes and in mouse liver.14 The mechanism for how caffeine inhibited mTORC1 was not investigated in this study. Intriguingly, caffeine treatment increased intracellular nucleotides such as NADH, ATP, AMP and ADP levels, possibly due to increased lipolysis and mitochondrial-β oxidation. Increased ATP levels normally result in inhibition of AMPK. Although the direct phosphorylation levels of AMPK were not determined, Sinha et al. found that caffeine treatment increased the ratio of phosphorylated acetyl-CoA carboxylase (p-ACC) vs. total ACC. However, the increased ratio of p-ACC/ACC might not necessarily reflect an increased AMPK activity because caffeine dramatically decreased total ACC levels by unknown mechanisms. Given the increased hepatic ATP levels and the decreased total ACC levels, it was less likely that caffeine induced inhibition of mTORC1 was AMPK dependent. In addition to AMPK, mTORC1 is positively regulated by the PI3K-AKT. Although it was not investigated in this study, it has been shown that caffeine inhibits AKT phosphorylation and in turn inhibits mTORC1 in non-hepatocytes.19 Therefore, future works are needed to determine the phosphorylation levels of AKT and AMPK to further dissect the mechanisms by which caffeine inhibited mTORC1 in hepatocytes. In addition to negatively regulating autophagy, mTORC1 positively regulates lipid biosynthesis. It will also be interesting to determine whether caffeine could also decrease gene expression of lipid synthesis genes in addition to induction of lipophagy.
Another intriguing finding in the studies by Sinha et al. was that caffeine increased expression of several autophagy proteins in hepatocytes, suggesting that caffeine may also regulate autophagy/lipophagy at the transcriptional level.14 Recently, it was found that the transcriptional factor EB (TFEB), a basic helix-loop-helix leucine zipper transcription factor of the Myc family, is a master regulator for controlling the expression of both autophagy and lysosomal genes. More importantly, over-expression of TFEB in mouse livers significantly inhibits diet-induced steatosis and obesity. In addition to regulating autophagy, TFEB also activates peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α) and peroxisome proliferator-activated receptor α (PPARα), two key transcriptional regulators for mitochondrial biogenesis and lipid catabolism.20 Interestingly, TFEB is regulated at both transcriptional and post-translational levels. TFEB itself is its own target gene, which perhaps can ensure removal of overloaded cellular lipids more efficiently by making more TFEB. The post-translational modification of TFEB mainly regulates its cellular location, and dephosphorylated TFEB translocates from cytosol to the nucleus. Three different kinases have been shown to phosphorylate TFEB: ERK2, mTOR and protein kinase Cβ. Although it was not studied in this study, caffeine might activate TFEB because of its inhibition of mTORC1. Activated TFEB might be responsible for the caffeine-induced lipophagy/lipolysis, mitochondrial bioenergetics and β-oxidation observed in this study.
Taken together, this important work expands our understanding of how caffeine benefits the liver functions through inducing lipophagy and mitochondrial β-oxidation. However, several important questions still need to be answered. How are LDs selectively recognized and removed by autophagy? Are other selective autophagy receptors, such as ubiquitin and p62/SQSTM1, also involved in selective lipophagy? What is the proper dose of caffeine in terms of drinking coffee to induce lipophagy, since overdose of caffeine may be detrimental to the cardiovascular system? Nevertheless, the fact that caffeine can not only induce lipophagy but also increase lipid mitochondrial β-oxidation suggests that drinking a couple cups of coffee per day may help to burn the fat out of your liver.
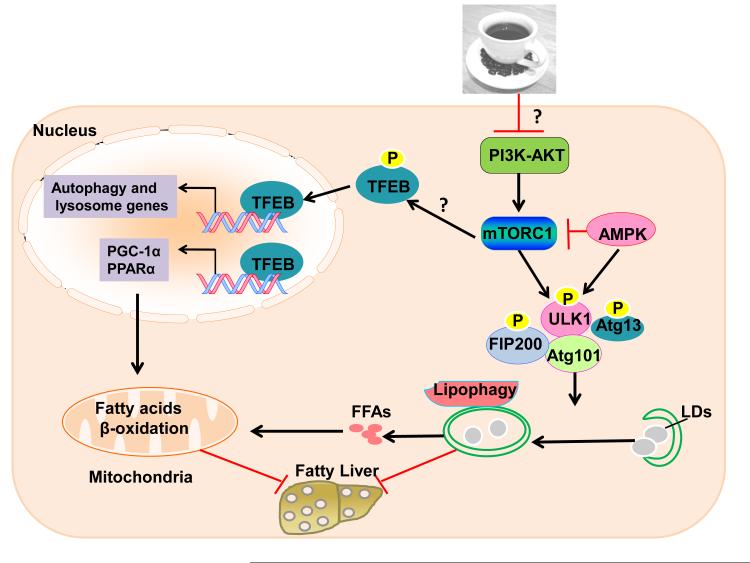
Figure 1. Proposed molecular signaling events in caffeine-induced lipophagy and mitochondria β-oxidation.
Caffeine may inhibit PI3-AKT and in turn inhibit mTOR to trigger autophagy by activating the ULK1 complex, which includes ULK1, Atg13, FIP200 and Atg101. Autophagy selectively removes excess lipid droplets (LDs) to generate free fatty acids (FFAs). Decreased mTOR induces TFEB nuclear translocation by decreasing TFEB phosphorylation. TFEB upregulates expression of autophagy and lysosomal genes, as well as PGC-1α and PPARα, which burns FFAs by increasing mitochondria β-oxidation. Thus, caffeine protects against fatty liver by coordinately inducing lipophagy and mitochondrial β-oxidation. “?” indicates molecular events that were not studied in this study.
Acknowledgments
Grant support: R01 AA020518 (W.X.D), and 5P20 RR021940-07 and 8 P20 GM103549-07 (Pharmacology, Toxicology and Therapeutics Department COBRE grant).
Abbreviations
- ACC
acetyl-CoA carboxylase
- AMPK
AMP-activated protein kinase
- FFAs
free fatty acids
- LDs
lipid droplets
- mTORC1
mammalian target of rapamycin complex 1
- TFEB
transcriptional factor EB
- TGF-β
transforming growth factor β
References
- 1.Liu J, Sui X, Lavie CJ, Hebert JR, Earnest CP, Zhang J, et al. Association of Coffee Consumption With All-Cause and Cardiovascular Disease Mortality. Mayo Clin Proc. 2013 doi: 10.1016/j.mayocp.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freedman ND, Park Y, Abnet CC, Hollenbeck AR, Sinha R. Association of coffee drinking with total and cause-specific mortality. N Engl J Med. 2012;366:1891–904. doi: 10.1056/NEJMoa1112010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruhl CE, Everhart JE. Coffee and caffeine consumption reduce the risk of elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2005;128:24–32. doi: 10.1053/j.gastro.2004.09.075. [DOI] [PubMed] [Google Scholar]
- 4.Molloy JW, Calcagno CJ, Williams CD, Jones FJ, Torres DM, Harrison SA. Association of coffee and caffeine consumption with fatty liver disease, nonalcoholic steatohepatitis, and degree of hepatic fibrosis. Hepatology. 2012;55:429–36. doi: 10.1002/hep.24731. [DOI] [PubMed] [Google Scholar]
- 5.Anty R, Marjoux S, Iannelli A, Patouraux S, Schneck AS, Bonnafous S, et al. Regular coffee but not espresso drinking is protective against fibrosis in a cohort mainly composed of morbidly obese European women with NAFLD undergoing bariatric surgery. J Hepatol. 2012;57:1090–6. doi: 10.1016/j.jhep.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Kalthoff S, Ehmer U, Freiberg N, Manns MP, Strassburg CP. Coffee induces expression of glucuronosyltransferases by the aryl hydrocarbon receptor and Nrf2 in liver and stomach. Gastroenterology. 2010;139:1699–710. 1710, e1–2. doi: 10.1053/j.gastro.2010.06.048. [DOI] [PubMed] [Google Scholar]
- 7.Gressner OA. Less Smad2 is good for you! A scientific update on coffee's liver benefits. Hepatology. 2009;50:970–8. doi: 10.1002/hep.23097. [DOI] [PubMed] [Google Scholar]
- 8.Gressner OA, Lahme B, Rehbein K, Siluschek M, Weiskirchen R, Gressner AM. Pharmacological application of caffeine inhibits TGF-beta-stimulated connective tissue growth factor expression in hepatocytes via PPARgamma and SMAD2/3-dependent pathways. J Hepatol. 2008;49:758–67. doi: 10.1016/j.jhep.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 9.Vitaglione P, Morisco F, Mazzone G, Amoruso DC, Ribecco MT, Romano A, et al. Coffee reduces liver damage in a rat model of steatohepatitis: the underlying mechanisms and the role of polyphenols and melanoidins. Hepatology. 2010;52:1652–61. doi: 10.1002/hep.23902. [DOI] [PubMed] [Google Scholar]
- 10.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, et al. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–5. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding WX, Li M, Chen X, Ni HM, Lin CW, Gao W, et al. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology. 2010;139:1740–52. doi: 10.1053/j.gastro.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin CW, Zhang H, Li M, Xiong X, Chen X, Dong XC, et al. Pharmacological promotion of autophagy alleviates steatosis and injury in alcoholic and non-alcoholic fatty liver conditions in mice. J Hepatol. 2013;58:993–9. doi: 10.1016/j.jhep.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuervo AM. Preventing lysosomal fat indigestion. Nat Cell Biol. 2013;15:565–7. doi: 10.1038/ncb2778. [DOI] [PubMed] [Google Scholar]
- 14.Sinha RA, Farah BL, Singh BK, Siddique MM, Li Y, Wu Y, et al. Caffeine stimulates hepatic lipid metabolism via autophagy-lysosomal pathway. Hepatology. 2013 doi: 10.1002/hep.26667. [DOI] [PubMed] [Google Scholar]
- 15.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–41. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–26. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–90. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 18.Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–61. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saiki S, Sasazawa Y, Imamichi Y, Kawajiri S, Fujimaki T, Tanida I, et al. Caffeine induces apoptosis by enhancement of autophagy via PI3K/Akt/mTOR/p70S6K inhibition. Autophagy. 2011;7:176–87. doi: 10.4161/auto.7.2.14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Settembre C, De Cegli R, Mansueto G, Saha PK, Vetrini F, Visvikis O, et al. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat Cell Biol. 2013;15:647–58. doi: 10.1038/ncb2718. [DOI] [PMC free article] [PubMed] [Google Scholar]